Comparison of 3 vaccination strategies against porcine reproductive and respiratory syndrome virus, Mycoplasma hyopneumoniae, and porcine circovirus type 2 on a 3 pathogen challenge model
- PMID: 29382967
- PMCID: PMC5764041
Comparison of 3 vaccination strategies against porcine reproductive and respiratory syndrome virus, Mycoplasma hyopneumoniae, and porcine circovirus type 2 on a 3 pathogen challenge model
Abstract
The objective of this study was to compare clinical, microbiologic, immunologic, and pathologic parameters in pigs each concurrently administered porcine reproductive and respiratory syndrome virus (PRRSV), Mycoplasma hyopneumoniae, and porcine circovirus type 2 (PCV2) vaccine from 1 of 2 commercial sources at 21 days of age and challenged with field strains of each of the 3 pathogens. Pigs were challenged with PRRSV and M. hyopneumoniae at 42 days of age (-14 days post-challenge, dpc) followed by a challenge with PCV2 at 56 days of age (0 dpc). Significant differences were observed between vaccinated challenged and unvaccinated challenged groups in clinical (average daily gain and clinical signs), microbiologic (viremia and nasal shedding), immunologic (antibodies and interferon-γ secreting cells), and pathologic (lesions) outcomes. Significant differences were observed among the 3 vaccinated challenged groups in microbiologic (nasal shedding of M. hyopneumoniae and viremia of PCV2) and immunologic (M. hyopneumoniae- and PCV2-specific interferon-γ secreting cells) outcomes. The vaccination regimen for PRRSV vaccine, M. hyopneumoniae vaccine, and PCV2 vaccine is efficacious for controlling triple challenge with PRRSV, M. hyopneumoniae, and PCV2 from weaning to finishing period.
L’objectif de la présente étude était de comparer les paramètres cliniques, microbiologiques, immunologiques et pathologiques chez des porcs qui ont chacun été vaccinés de façon concomitante à 21 jours d’âge contre le virus du syndrome reproducteur et respiratoire porcin (VSRRP), Mycoplasma hyopneumoniae, et le circovirus porcin de type 2 (CVP2) provenant d’une de deux sources commerciales et soumis à une infection défi avec des souches de champs de chacun des trois agents pathogènes. Les porcs ont été infectés avec le VSRRP et M. hyopneumoniae à 42 jours d’âge (−14 jours post-infection, jpi) suivi d’une infection par le CVP2 à 56 jours d’âge (0 jpi). Des différences significatives ont été observées entre les groupes vaccinés et infectés et les groupes non-vaccinés infectés pour ce qui est résultats cliniques (gain moyen quotidien et signes cliniques), microbiologiques (virémie et excrétion nasale), immunologiques (anticorps et cellules secrétant de l’interféron-γ), et pathologiques (lésions). Des différences significatives ont été observées entre les trois groupes d’animaux vaccinés et infectés pour ce qui est des résultats microbiologiques (excrétion nasale de M. hyopneumoniae et virémie de CVP2) et immunologiques (cellules secrétant de l’interféron-γ spécifiques à M. hyopneumoniae et CVP2). Le protocole de vaccination pour le vaccin VSRRP, le vaccin M. hyopneumoniae, et le vaccin CVP2 est efficace pour la maitrise d’une infection triple avec le VSRRP, M. hyopneumoniae, et CVP2 à partir du sevrage jusqu’à la période de finition.(Traduit par Docteur Serge Messier).
Figures
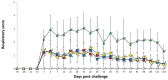
 ), Zoetis-VacB/Ch (
), Zoetis-VacB/Ch (
 ), BI-Vac/Ch (
), BI-Vac/Ch (
 ), UnVac/Ch (
), UnVac/Ch (
 ), and UnVac/UnCh (⋄). Different letters within a sampling point mean statistically significant differences (P < 0.05).
), and UnVac/UnCh (⋄). Different letters within a sampling point mean statistically significant differences (P < 0.05).
 ), Zoetis-VacB/Ch (
), Zoetis-VacB/Ch (
 ), BI-Vac/Ch (
), BI-Vac/Ch (
 ), UnVac/Ch (
), UnVac/Ch (
 ), and UnVac/UnCh (⋄). Different letters within a sampling point mean statistically significant differences (P < 0.05).
), and UnVac/UnCh (⋄). Different letters within a sampling point mean statistically significant differences (P < 0.05).
 ), Zoetis-VacB/Ch (
), Zoetis-VacB/Ch (
 ), BI-Vac/Ch (
), BI-Vac/Ch (
 ), UnVac/Ch (
), UnVac/Ch (
 ), and UnVac/UnCh (⋄). Different letters within a sampling point mean statistically significant differences (P < 0.05).
), and UnVac/UnCh (⋄). Different letters within a sampling point mean statistically significant differences (P < 0.05).
 ), Zoetis-VacB/Ch (
), Zoetis-VacB/Ch (
 ), BI-Vac/Ch (
), BI-Vac/Ch (
 ), UnVac/Ch (
), UnVac/Ch (
 ), and UnVac/UnCh (⋄). Different letters within a sampling point mean statistically significant differences (P < 0.05).
), and UnVac/UnCh (⋄). Different letters within a sampling point mean statistically significant differences (P < 0.05).References
-
- Chae C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet J. 2016;212:1–6. - PubMed
-
- Thacker EL. Porcine respiratory disease complex — What is and why does it remain a problem? Pig J. 2001;48:66–70.
-
- Kim J, Chung H-K, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. 2003;166:251–256. - PubMed
-
- Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169:326–336. - PubMed
-
- Chae C. Porcine circovirus type 2 and its associated disease in Korea. Virus Res. 2012;164:107–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
