Snail1-expressing cancer-associated fibroblasts induce lung cancer cell epithelial-mesenchymal transition through miR-33b
- PMID: 29383119
- PMCID: PMC5777731
- DOI: 10.18632/oncotarget.23082
Snail1-expressing cancer-associated fibroblasts induce lung cancer cell epithelial-mesenchymal transition through miR-33b
Abstract
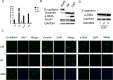
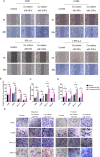
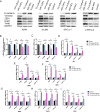
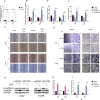
Lung cancer has a high propensity for metastasis. Cancer-associated fibroblasts (CAFs) are the main type of stromal cells in cancer tissue, are activated by tumor cells, and play a significant role in tumor development. However, whether CAFs induce lung cancer cell metastasis, as well as pathway involved in CAF-induced lung cancer cell metastasis, is uncertain. Snail1 is a transcriptional factor whose expression in the stroma is associated with lower survival rates in patients with cancer. However, how Snail1 regulates the crosstalk between stromal cells and tumor cells when it is expressed in the stroma has not been determined. Altered microRNA (miRNA) expression is correlated with lung cancer metastasis. Our previous study of microRNAs showed that miR-33b levels were clearly reduced in lung cancer cell lines and lung cancer tissues, and miR-33b suppressed tumor cell epithelial-mesenchymal transition (EMT) when its expression was elevated. In this study, we found that co-culturing CAFs with lung cancer cells induced miR-33b downregulation and promoted epithelial cells EMT. Moreover, we found that miR-33b overexpression in lung cancer cells counteracted CAF-induced EMT. Interestingly, Snail1 expression in fibroblasts activate the inductive effects of CAFs on lung cancer cell EMT. Hence, understanding the molecular mechanism underlying the communication between stromal cells and tumor cells mediated by miR-33b may lead to the identification of novel targets for the treatment of lung cancer. Additionally, understanding the role of Snail1 driving CAFs to induce lung cancer cell EMT may provide with a new perspective on the treatment of lung cancer.
Keywords: Snail1; cancer-associated fibroblasts; epithelial-mesenchymal transition; lung cancer; microRNA.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures




References
-
- Field JK, Oudkerk M, Pedersen JH, Duffy SW. Prospects for population screening and diagnosis of lung cancer. Lancet. 2013;382:732–741. - PubMed
-
- Curioni-Fontecedro A, Husmann L, Soldini D, Stahel RA. Primary non-small cell lung cancer response upon treatment with denosumab. Lung Cancer. 2013;82:506–508. - PubMed
-
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. - PubMed
-
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

