Hypoxia-activated prodrug enhances therapeutic effect of sunitinib in melanoma
- PMID: 29383148
- PMCID: PMC5777760
- DOI: 10.18632/oncotarget.22944
Hypoxia-activated prodrug enhances therapeutic effect of sunitinib in melanoma
Abstract
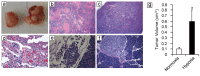
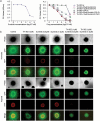
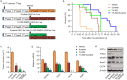
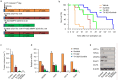
Angiogenesis is a critical step during tumor progression. Anti-angiogenic therapy has only provided modest benefits in delaying tumor progression despite its early promise in cancer treatment. It has been postulated that anti-angiogenic therapy may promote the emergence of a more aggressive cancer cell phenotype by generating increased tumor hypoxia-a well-recognized promoter of tumor progression. TH-302 is a 2-nitroimidazole triggered hypoxia-activated prodrug (HAP) which has been shown to selectively target the hypoxic tumor compartment and reduce tumor volume. Here, we show that melanoma cells grown under hypoxic conditions exhibit increased resistance to a wide variety of therapeutic agents in vitro and generate larger and more aggressive tumors in vivo than melanoma cells grown under normoxic conditions. However, hypoxic melanoma cells exhibit a pronounced sensitivity to TH-302 which is further enhanced by the addition of sunitinib. Short term sunitinib treatment fails to prolong the survival of melanoma bearing genetically engineered mice (Tyr::CreER; BRafCA;Ptenlox/lox ) but increases tumor hypoxia. Long term TH-302 alone modestly prolongs the overall survival of melanoma bearing mice. Combination therapy of TH-302 with sunitinib further increases the survival of treated mice. These studies provide a translational rationale for combining hypoxic tumor cell targeted therapies with anti-angiogenics for treatment of melanoma.
Keywords: TH302; hypoxia; melanoma; sunitinib; treatment.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures



References
-
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. - PubMed
-
- Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215–236. - PubMed
-
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, et al. Final version of the american joint committee on cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. - PubMed
-
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, Atkins MB, Thompson JA, Coit DG, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the american joint committee on cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. - PubMed
-
- La Porta CA. Mechanism of drug sensitivity and resistance in melanoma. Curr Cancer Drug Targets. 2009;9:391–397. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

