Ca2+ signalling in mouse urethral smooth muscle in situ: role of Ca2+ stores and Ca2+ influx mechanisms
- PMID: 29383731
- PMCID: PMC5899989
- DOI: 10.1113/JP275719
Ca2+ signalling in mouse urethral smooth muscle in situ: role of Ca2+ stores and Ca2+ influx mechanisms
Abstract
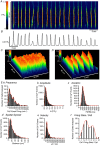
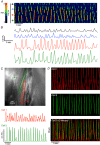
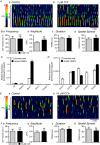
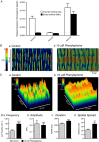
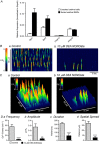
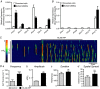
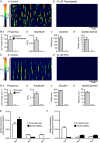
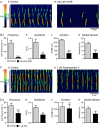
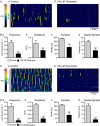
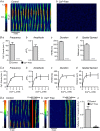
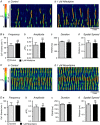
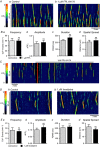
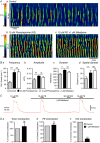
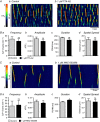
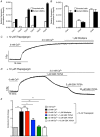
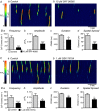
Key points: Contraction of urethral smooth muscle cells (USMCs) contributes to urinary continence. Ca2+ signalling in USMCs was investigated in intact urethral muscles using a genetically encoded Ca2+ sensor, GCaMP3, expressed selectively in USMCs. USMCs were spontaneously active in situ, firing intracellular Ca2+ waves that were asynchronous at different sites within cells and between adjacent cells. Spontaneous Ca2+ waves in USMCs were myogenic but enhanced by adrenergic or purinergic agonists and decreased by nitric oxide. Ca2+ waves arose from inositol trisphosphate type 1 receptors and ryanodine receptors, and Ca2+ influx by store-operated calcium entry was required to maintain Ca2+ release events. Ca2+ release and development of Ca2+ waves appear to be the primary source of Ca2+ for excitation-contraction coupling in the mouse urethra, and no evidence was found that voltage-dependent Ca2+ entry via L-type or T-type channels was required for responses to α adrenergic responses.
Abstract: Urethral smooth muscle cells (USMCs) generate myogenic tone and contribute to urinary continence. Currently, little is known about Ca2+ signalling in USMCs in situ, and therefore little is known about the source(s) of Ca2+ required for excitation-contraction coupling. We characterized Ca2+ signalling in USMCs within intact urethral muscles using a genetically encoded Ca2+ sensor, GCaMP3, expressed selectively in USMCs. USMCs fired spontaneous intracellular Ca2+ waves that did not propagate cell-to-cell across muscle bundles. Ca2+ waves increased dramatically in response to the α1 adrenoceptor agonist phenylephrine (10 μm) and to ATP (10 μm). Ca2+ waves were inhibited by the nitric oxide donor DEA NONOate (10 μm). Ca2+ influx and release from sarcoplasmic reticulum stores contributed to Ca2+ waves, as Ca2+ free bathing solution and blocking the sarcoplasmic Ca2+ -ATPase abolished activity. Intracellular Ca2+ release involved cooperation between ryanadine receptors and inositol trisphosphate receptors, as tetracaine and ryanodine (100 μm) and xestospongin C (1 μm) reduced Ca2+ waves. Ca2+ waves were insensitive to L-type Ca2+ channel modulators nifedipine (1 μm), nicardipine (1 μm), isradipine (1 μm) and FPL 64176 (1 μm), and were unaffected by the T-type Ca2+ channel antagonists NNC-550396 (1 μm) and TTA-A2 (1 μm). Ca2+ waves were reduced by the store operated Ca2+ entry blocker SKF 96365 (10 μm) and by an Orai antagonist, GSK-7975A (1 μm). The latter also reduced urethral contractions induced by phenylephrine, suggesting that Orai can function effectively as a receptor-operated channel. In conclusion, Ca2+ waves in mouse USMCs are a source of Ca2+ for excitation-contraction coupling in urethral muscles.
Keywords: Ca2+ imaging; lower urinary tract; optogenetics; store-operated Ca2+ entry; urinary continence.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures

Similar articles
-
Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells of the rabbit urethra in situ.J Physiol. 2007 Sep 1;583(Pt 2):505-19. doi: 10.1113/jphysiol.2007.136697. Epub 2007 Jul 5. J Physiol. 2007. PMID: 17615099 Free PMC article.
-
Contribution of Cav1.2 Ca2+ channels and store-operated Ca2+ entry to pig urethral smooth muscle contraction.Am J Physiol Renal Physiol. 2020 Feb 1;318(2):F496-F505. doi: 10.1152/ajprenal.00514.2019. Epub 2020 Jan 6. Am J Physiol Renal Physiol. 2020. PMID: 31904286
-
Interstitial cell of Cajal-like cells (ICC-LC) exhibit dynamic spontaneous activity but are not functionally innervated in mouse urethra.Cell Calcium. 2024 Nov;123:102931. doi: 10.1016/j.ceca.2024.102931. Epub 2024 Jul 22. Cell Calcium. 2024. PMID: 39068674
-
Store-operated calcium channels: Potential target for the therapy of hypertension.Rev Cardiovasc Med. 2019 Sep 30;20(3):139-151. doi: 10.31083/j.rcm.2019.03.522. Rev Cardiovasc Med. 2019. PMID: 31601088 Review.
-
Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle.Clin Exp Pharmacol Physiol. 2008 Sep;35(9):1127-33. doi: 10.1111/j.1440-1681.2008.05018.x. Clin Exp Pharmacol Physiol. 2008. PMID: 18782202 Free PMC article. Review.
Cited by
-
Ca2+ -activated Cl- channels (TMEM16A) underlie spontaneous electrical activity in isolated mouse corpus cavernosum smooth muscle cells.Physiol Rep. 2022 Nov;10(22):e15504. doi: 10.14814/phy2.15504. Physiol Rep. 2022. PMID: 36394209 Free PMC article.
-
Calcium-Activated Chloride Channels in Myometrial and Vascular Smooth Muscle.Front Physiol. 2021 Oct 15;12:751008. doi: 10.3389/fphys.2021.751008. eCollection 2021. Front Physiol. 2021. PMID: 34867456 Free PMC article. Review.
-
Laboratory practical to study the differential innervation pathways of urinary tract smooth muscle.Adv Physiol Educ. 2018 Jun 1;42(2):295-304. doi: 10.1152/advan.00014.2018. Adv Physiol Educ. 2018. PMID: 29676616 Free PMC article.
-
Applications of Spatio-temporal Mapping and Particle Analysis Techniques to Quantify Intracellular Ca2+ Signaling In Situ.J Vis Exp. 2019 Jan 7;(143):10.3791/58989. doi: 10.3791/58989. J Vis Exp. 2019. PMID: 30663707 Free PMC article.
-
The intracellular Ca2+ release channel TRPML1 regulates lower urinary tract smooth muscle contractility.Proc Natl Acad Sci U S A. 2020 Dec 1;117(48):30775-30786. doi: 10.1073/pnas.2016959117. Epub 2020 Nov 16. Proc Natl Acad Sci U S A. 2020. PMID: 33199609 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

