Circadian expression and functional characterization of PEA-15 within the mouse suprachiasmatic nucleus
- PMID: 29383758
- PMCID: PMC5880735
- DOI: 10.1111/ejn.13850
Circadian expression and functional characterization of PEA-15 within the mouse suprachiasmatic nucleus
Abstract
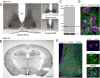
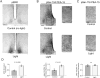
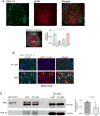
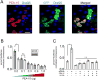
The circadian timing system influences the functional properties of most, if not all, physiological processes. Central to the mammalian timing system is the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN functions as a 'master clock' that sets the phasing of ancillary circadian oscillator populations found throughout the body. Further, via an entraining input from the retina, the SCN ensures that the clock oscillators are synchronized to the daily light/dark cycle. A critical component of the SCN timing and entrainment systems is the p44/42 mitogen-activated protein kinase (ERK/MAPK) pathway. Here, we examined the expression and function of phosphoprotein-enriched in astrocytes (PEA-15), an ERK scaffold protein that serves as a key regulator of MAPK signaling. A combination of immunolabeling and Western blotting approaches revealed high levels of PEA-15 within the SCN. PEA-15 expression was enriched in distinct subpopulations of SCN neurons, including arginine vasopressin (AVP)-positive neurons of the SCN shell region. Further, expression profiling detected a significant circadian oscillation in PEA-15 expression within the SCN. Brief photic stimulation during the early subjective night led to a significant increase in PEA-15 phosphorylation, an event that can trigger ERK/PEA-15 dissociation. Consistent with this, co-immunoprecipitation assays revealed that PEA-15 is directly bound to ERK in the SCN and that photic stimulation leads to their dissociation. Finally, we show that PEA-15 regulates ERK/MAPK-dependent activation of the core clock gene period1. Together, these data raise the prospect that PEA-15 functions as a key regulator of the SCN timing system.
Keywords: ERK; C57Bl/6; PEA-15; circadian; suprachiasmatic nucleus.
© 2018 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
SynGAP is expressed in the murine suprachiasmatic nucleus and regulates circadian-gated locomotor activity and light-entrainment capacity.Eur J Neurosci. 2021 Feb;53(3):732-749. doi: 10.1111/ejn.15043. Epub 2020 Nov 30. Eur J Neurosci. 2021. PMID: 33174316 Free PMC article.
-
Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism.J Neurosci. 2005 Jun 1;25(22):5305-13. doi: 10.1523/JNEUROSCI.4361-04.2005. J Neurosci. 2005. PMID: 15930378 Free PMC article.
-
Mitogen- and stress-activated protein kinase 1 modulates photic entrainment of the suprachiasmatic circadian clock.Eur J Neurosci. 2013 Jan;37(1):130-40. doi: 10.1111/ejn.12028. Epub 2012 Nov 6. Eur J Neurosci. 2013. PMID: 23127194 Free PMC article.
-
[Mechanisms of structural plasticity associated with photic synchronization of the circadian clock within the suprachiasmatic nucleus].J Soc Biol. 2009;203(1):49-63. doi: 10.1051/jbio:2009004. Epub 2009 Apr 10. J Soc Biol. 2009. PMID: 19358811 Review. French.
-
Neuroglial and synaptic rearrangements associated with photic entrainment of the circadian clock in the suprachiasmatic nucleus.Eur J Neurosci. 2010 Dec;32(12):2133-42. doi: 10.1111/j.1460-9568.2010.07520.x. Eur J Neurosci. 2010. PMID: 21143667 Review.
Cited by
-
SynGAP is expressed in the murine suprachiasmatic nucleus and regulates circadian-gated locomotor activity and light-entrainment capacity.Eur J Neurosci. 2021 Feb;53(3):732-749. doi: 10.1111/ejn.15043. Epub 2020 Nov 30. Eur J Neurosci. 2021. PMID: 33174316 Free PMC article.
-
PEA-15 C-Terminal Tail Allosterically Modulates Death-Effector Domain Conformation and Facilitates Protein-Protein Interactions.Int J Mol Sci. 2019 Jul 7;20(13):3335. doi: 10.3390/ijms20133335. Int J Mol Sci. 2019. PMID: 31284641 Free PMC article.
References
-
- Agostino PV, Ferreyra GA, Murad AD, Watanabe Y, Golombek DA. Diurnal, circadian and photic regulation of calcium/calmodulin-dependent kinase II and neuronal nitric oxide synthase in the hamster suprachiasmatic nuclei. Neurochem Int. 2004;44:617–625. - PubMed
-
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19:1115–1121. - PMC - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. - PubMed
-
- Araujo H, Danziger N, Cordier J, Glowinski J, Chneiweiss H. Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J Biol Chem. 1993;268:5911–5920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

