Interplay between Trx-1 and S100P promotes colorectal cancer cell epithelial-mesenchymal transition by up-regulating S100A4 through AKT activation
- PMID: 29383839
- PMCID: PMC5867135
- DOI: 10.1111/jcmm.13541
Interplay between Trx-1 and S100P promotes colorectal cancer cell epithelial-mesenchymal transition by up-regulating S100A4 through AKT activation
Abstract
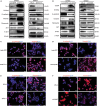
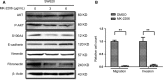
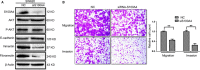
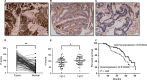
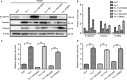
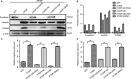
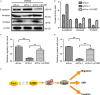
We previously reported a novel positive feedback loop between thioredoxin-1 (Trx-1) and S100P, which promotes the invasion and metastasis of colorectal cancer (CRC). However, the underlying molecular mechanisms remain poorly understood. In this study, we examined the roles of Trx-1 and S100P in CRC epithelial-to-mesenchymal transition (EMT) and their underlying mechanisms. We observed that knockdown of Trx-1 or S100P in SW620 cells inhibited EMT, whereas overexpression of Trx-1 or S100P in SW480 cells promoted EMT. Importantly, S100A4 and the phosphorylation of AKT were identified as potential downstream targets of Trx-1 and S100P in CRC cells. Silencing S100A4 or inhibition of AKT phosphorylation eliminated S100P- or Trx-1-mediated CRC cell EMT, migration and invasion. Moreover, inhibition of AKT activity reversed S100P- or Trx-1-induced S100A4 expression. The expression of S100A4 was higher in human CRC tissues compared with their normal counterpart tissues and was significantly correlated with lymph node metastasis and poor survival. The overexpression of S100A4 protein was also positively correlated with S100P or Trx-1 protein overexpression in our cohort of CRC tissues. In addition, overexpression of S100P reversed the Trx-1 knockdown-induced inhibition of S100A4 expression, EMT and migration and invasion in SW620 cells. The data suggest that interplay between Trx-1 and S100P promoted CRC EMT as well as migration and invasion by up-regulating S100A4 through AKT activation, thus providing further potential therapeutic targets for suppressing the EMT in metastatic CRC.
Keywords: S100A4; S100P; colorectal cancer; epithelial-mesenchymal transition; thioredoxin-1.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
Thioredoxin-1 promotes colorectal cancer invasion and metastasis through crosstalk with S100P.Cancer Lett. 2017 Aug 10;401:1-10. doi: 10.1016/j.canlet.2017.04.036. Epub 2017 May 5. Cancer Lett. 2017. PMID: 28483515
-
The emerging role of S100A4 and S100A14 proteins in colorectal cancer progression.Cell Mol Biol (Noisy-le-grand). 2024 Nov 27;70(11):134-143. doi: 10.14715/cmb/2024.70.11.20. Cell Mol Biol (Noisy-le-grand). 2024. PMID: 39707769
-
miR-296 inhibits the metastasis and epithelial-mesenchymal transition of colorectal cancer by targeting S100A4.BMC Cancer. 2017 Feb 16;17(1):140. doi: 10.1186/s12885-017-3121-z. BMC Cancer. 2017. PMID: 28209128 Free PMC article.
-
Research Progress of Epithelial-mesenchymal Transition Treatment and Drug Resistance in Colorectal Cancer.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221081219. doi: 10.1177/15330338221081219. Technol Cancer Res Treat. 2022. PMID: 35435774 Free PMC article. Review.
-
Pharmacological Targeting of Epithelial-to-Mesenchymal Transition in Colorectal Cancer.Curr Pharm Des. 2022;28(28):2298-2311. doi: 10.2174/1381612828666220728152350. Curr Pharm Des. 2022. PMID: 35909286 Review.
Cited by
-
Prognostic Value of S100P Expression in Patients With Digestive System Cancers: A Meta-Analysis.Front Oncol. 2021 Mar 5;11:593728. doi: 10.3389/fonc.2021.593728. eCollection 2021. Front Oncol. 2021. PMID: 33747914 Free PMC article.
-
The Role of Long Non-Coding RNAs in Thyroid Cancer.Front Oncol. 2020 Jun 11;10:941. doi: 10.3389/fonc.2020.00941. eCollection 2020. Front Oncol. 2020. PMID: 32596158 Free PMC article. Review.
-
Thioredoxin 1 supports colorectal cancer cell survival and promotes migration and invasion under glucose deprivation through interaction with G6PD.Int J Biol Sci. 2022 Aug 29;18(14):5539-5553. doi: 10.7150/ijbs.71809. eCollection 2022. Int J Biol Sci. 2022. PMID: 36147458 Free PMC article.
-
Compensatory Protection of Thioredoxin-Deficient Cells from Etoposide-Induced Cell Death by Selenoprotein W via Interaction with 14-3-3.Int J Mol Sci. 2021 Sep 25;22(19):10338. doi: 10.3390/ijms221910338. Int J Mol Sci. 2021. PMID: 34638679 Free PMC article.
-
Identification of 8 candidate microsatellite instability loci in colorectal cancer and validation of the ACVR2A mechanism in the tumor progression.Sci Rep. 2024 Jun 19;14(1):14145. doi: 10.1038/s41598-024-62753-1. Sci Rep. 2024. PMID: 38898042 Free PMC article.
References
-
- Khair G, Monson JR, Greenman J. Epithelial molecular markers in the peripheral blood of patients with colorectal cancer. Dis Colon Rectum. 2007;50:1188‐1203. - PubMed
-
- Cao H, Xu E, Liu H, et al. Epithelial‐mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract. 2015;211:557‐569. - PubMed
-
- Zhao L, Li W, Zhou Y, et al. The overexpression and nuclear translocation of Trx‐1 during hypoxia confers on HepG2 cells resistance to DDP, and GL‐V9 reverses the resistance by suppressing the Trx‐1/Ref‐1 axis. Free Radic Biol Med. 2015;82:29‐41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

