Recent developments on triazole nucleus in anticonvulsant compounds: a review
- PMID: 29383949
- PMCID: PMC6010125
- DOI: 10.1080/14756366.2017.1423068
Recent developments on triazole nucleus in anticonvulsant compounds: a review
Abstract
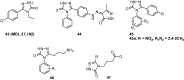
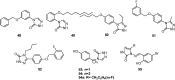
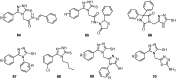
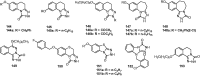
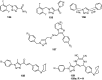
Epilepsy is one of the common diseases seriously threatening life and health of human. More than 50 million people are suffering from this condition and anticonvulsant agents are the main treatment. However, side effects and intolerance, and a lack of efficacy limit the application of the current anticonvulsant agents. The search for new anticonvulsant agents with higher efficacy and lower toxicity continues to be the focus and task in medicinal chemistry. Numbers of triazole derivatives as clinical drugs or candidates have been frequently employed for the treatment of various types of diseases, which have proved the importance of this heterocyclic nucleus in drug design and discovery. Recently many endeavours were made to involve the triazole into the anticonvulsants design, which have brought lots of active compounds. This work is an attempt to systematically review the research of triazole derivatives in the design and development of anticonvulsant agents during the past two decades.
Keywords: Epilepsy; MES; anticonvulsant; scPTZ; triazole.
Figures
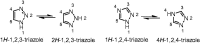
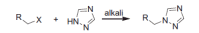
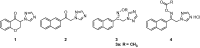

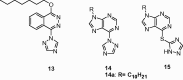
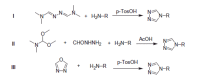
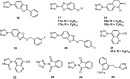
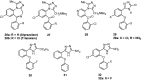

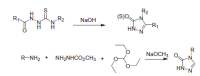


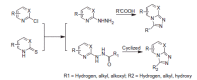


Similar articles
-
1,2,4-triazole derivatives as potential scaffold for anticonvulsant activity.Cent Nerv Syst Agents Med Chem. 2015;15(1):17-22. doi: 10.2174/1871524915666150209100533. Cent Nerv Syst Agents Med Chem. 2015. PMID: 25675400 Review.
-
The importance of triazole scaffold in the development of anticonvulsant agents.Eur J Med Chem. 2016 Feb 15;109:380-92. doi: 10.1016/j.ejmech.2016.01.009. Epub 2016 Jan 11. Eur J Med Chem. 2016. PMID: 26826582 Review.
-
An Important Potential Anti-Epileptic/Anticonvulsant Active Group: A Review of 1,2,4-Triazole Groups and Their Action.Drug Res (Stuttg). 2022 Mar;72(3):131-138. doi: 10.1055/a-1670-6992. Epub 2021 Nov 10. Drug Res (Stuttg). 2022. PMID: 34758502 Review.
-
3,4-DHQLO and Triazole and Its Related Analogues with Anticonvulsant Effects.Mini Rev Med Chem. 2016;16(4):323-42. doi: 10.2174/1389557515666150101100909. Mini Rev Med Chem. 2016. PMID: 25553427 Review.
-
Triazole incorporated thiazoles as a new class of anticonvulsants: design, synthesis and in vivo screening.Eur J Med Chem. 2010 Apr;45(4):1536-43. doi: 10.1016/j.ejmech.2009.12.062. Epub 2010 Jan 13. Eur J Med Chem. 2010. PMID: 20116140
Cited by
-
Synthesis, cytotoxicity and 99mTc-MIBI tumor cell uptake evaluation of 2-phenylbenzothiazole tagged triazole derivatives.Future Med Chem. 2024;16(19):1999-2012. doi: 10.1080/17568919.2024.2389771. Epub 2024 Sep 4. Future Med Chem. 2024. PMID: 39229781
-
Effect of Chronic Administration of 5-(3-chlorophenyl)-4-Hexyl-2,4 -Dihydro-3H-1,2,4-Triazole-3-Thione (TP-315)-A New Anticonvulsant Drug Candidate-On Living Organisms.Int J Mol Sci. 2021 Mar 25;22(7):3358. doi: 10.3390/ijms22073358. Int J Mol Sci. 2021. PMID: 33805962 Free PMC article.
-
Design, synthesis, and anticonvulsant effects evaluation of nonimidazole histamine H3 receptor antagonists/inverse agonists containing triazole moiety.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1310-1321. doi: 10.1080/14756366.2020.1774573. J Enzyme Inhib Med Chem. 2020. PMID: 32529860 Free PMC article.
-
Design, synthesis, and in vitro, in vivo, and in silico evaluation of novel substituted 1,3,4-thiadiazole derivatives as anticonvulsant agents.Front Chem. 2025 Feb 12;12:1515866. doi: 10.3389/fchem.2024.1515866. eCollection 2024. Front Chem. 2025. PMID: 40012830 Free PMC article.
-
One-pot parallel synthesis of 1,3,5-trisubstituted 1,2,4-triazoles.Mol Divers. 2022 Apr;26(2):993-1004. doi: 10.1007/s11030-021-10218-2. Epub 2021 Apr 2. Mol Divers. 2022. PMID: 33797670 Free PMC article.
References
-
- Chang BS, Lowenstein DH.. Epilepsy. N Engl J Med 2003;349:1257–66. - PubMed
-
- Singh SP, Sankaraneni R, Antony AR.. Evidence-based guidelines for the management of epilepsy. Neurol India 2017;65:S6–S11. - PubMed
-
- Terrone G, Pauletti A, Pascente R, et al. . Preventing epileptogenesis: a realistic goal? Pharmacol Res 2016;110:96–100. - PubMed
-
- Stephen JM, Hammer GD, eds. Pathophysiology of disease: an introduction to clinical medicine. 6th ed New York: McGraw-Hill; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
