Prevalence and Management of Drug-Related Problems in Chronic Kidney Disease Patients by Severity Level: A Subanalysis of a Cluster Randomized Controlled Trial in Community Pharmacies
- PMID: 29384023
- PMCID: PMC10398068
- DOI: 10.18553/jmcp.2018.24.2.173
Prevalence and Management of Drug-Related Problems in Chronic Kidney Disease Patients by Severity Level: A Subanalysis of a Cluster Randomized Controlled Trial in Community Pharmacies
Abstract
Background: Drug-related problems (DRPs) are prevalent among chronic kidney disease (CKD) patients. However, little is known about their severity and management by community pharmacists.
Objectives: To (a) describe the prevalence of DRPs by severity level in CKD patients and (b) assess the effect of a training-and-communication network program in nephrology (ProFiL) on these DRPs.
Methods: This is a secondary analysis of a cluster randomized controlled trial evaluating the effect of the ProFiL-program. In 6 CKD clinics, patients at CKD stage 3 or 4 and their community pharmacists were recruited and assigned to the ProFiL group or a usual care (UC) group. Using validated criteria, 2 pharmacists identified DRPs and assessed their severity at baseline and after 12 months. The mean annual change in the number of DRPs per patient by severity level was assessed using a 2-level multivariable linear mixed-effects model.
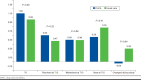
Results: A total of 494 pharmacists and 442 patients participated. At baseline, the prevalence (mean number of DRPs per patient [SD]) of mild DRPs (e.g., requiring dosage adjustment) and moderate DRPs (e.g., drug adherence requiring a monitoring plan) were 0.55 (0.98) and 1.04 (1.51), respectively. After 12 months, an unadjusted incremental annual reduction of 0.34 moderate DRPs (95% CI = -0.66 to -0.01) was observed in the ProFiL group compared with the UC group. After adjustment, no between-group differences were observed.
Conclusions: Among patients followed in CKD clinics, most DRPs have a moderate severity requiring specific monitoring by pharmacists. The benefit of continuing education programs, such as ProFiL, to reduce moderate DRPs remains to be determined.
Disclosures: This study was supported by the Canadian Institutes of Health Research (grant number: MOP-230207). Part of the study was also funded by Pfizer Canada, Leo Pharma, and Amgen. The authors declare that they have no relevant financial interests. Study concept and design were contributed by Quintana-Bárcena, Lord, and Lalonde. Quintana-Bárcena, Lord, and Lizotte were responsible for the data analysis, and Quintana-Bárcena and Berbiche performed the statistical analysis. The manuscript was written by Quintana-Bárcena and Lalonde and revised by Quintana-Bárcena and Lalonde, along with the other authors.
Conflict of interest statement
This study was supported by the Canadian Institutes of Health Research (grant number: MOP-230207). Part of the study was also funded by Pfizer Canada, Leo Pharma, and Amgen. The authors declare that they have no relevant financial interests.
Study concept and design were contributed by Quintana-Bárcena, Lord, and Lalonde. Quintana-Bárcena, Lord, and Lizotte were responsible for the data analysis, and Quintana-Bárcena and Berbiche performed the statistical analysis. The manuscript was written by Quintana-Bárcena and Lalonde and revised by Quintana-Bárcena and Lalonde, along with the other authors.
Figures
Similar articles
-
Community Pharmacist Training-and-Communication Network and Drug-Related Problems in Patients With CKD: A Multicenter, Cluster-Randomized, Controlled Trial.Am J Kidney Dis. 2017 Sep;70(3):386-396. doi: 10.1053/j.ajkd.2017.05.008. Epub 2017 Jun 26. Am J Kidney Dis. 2017. PMID: 28663062 Clinical Trial.
-
Evaluation of a training and communication-network nephrology program for community pharmacists.Pharm World Sci. 2008 Dec;30(6):924-33. doi: 10.1007/s11096-008-9253-0. Epub 2008 Sep 19. Pharm World Sci. 2008. PMID: 18802782 Clinical Trial.
-
Clinical medication reviews in elderly patients with polypharmacy: a cross-sectional study on drug-related problems in the Netherlands.Int J Clin Pharm. 2016 Feb;38(1):46-53. doi: 10.1007/s11096-015-0199-8. Epub 2015 Nov 23. Int J Clin Pharm. 2016. PMID: 26597955 Free PMC article.
-
Identification, classification, and documentation of drug related problems in community pharmacy practice in Europe: a scoping review.Int J Clin Pharm. 2025 Apr;47(2):247-269. doi: 10.1007/s11096-024-01834-7. Epub 2025 Jan 8. Int J Clin Pharm. 2025. PMID: 39775382 Free PMC article.
-
Nurse-led medication management as a critical component of transitional care for preventing drug-related problems.Aging Clin Exp Res. 2024 Jul 26;36(1):151. doi: 10.1007/s40520-024-02799-3. Aging Clin Exp Res. 2024. PMID: 39060872 Free PMC article. Review.
Cited by
-
Utilization of potentially inappropriate medication and risk of adverse drug events among older adults with chronic renal insufficiency: a population-wide cohort study.BMC Geriatr. 2021 Feb 10;21(1):117. doi: 10.1186/s12877-021-02057-5. BMC Geriatr. 2021. PMID: 33568102 Free PMC article.
-
Comparison of Drug-Related Problems in COVID-19 and Non-COVID-19 Patients Provided by a German Telepharmacy Service for Rural Intensive Care Units.J Clin Med. 2023 Jul 18;12(14):4739. doi: 10.3390/jcm12144739. J Clin Med. 2023. PMID: 37510855 Free PMC article.
-
Drug-related problems and associated factors among patients with kidney dysfunction at a tertiary hospital in southwestern Uganda: a prospective observational study.BMC Nephrol. 2023 Dec 19;24(1):375. doi: 10.1186/s12882-023-03437-2. BMC Nephrol. 2023. PMID: 38114948 Free PMC article.
-
Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease.BMC Geriatr. 2020 Mar 4;20(1):87. doi: 10.1186/s12877-020-1485-4. BMC Geriatr. 2020. PMID: 32131742 Free PMC article.
-
Drug-related problems in hospitalized patients with chronic kidney diseases and clinical pharmacist interventions.BMC Geriatr. 2023 Dec 13;23(1):849. doi: 10.1186/s12877-023-04557-y. BMC Geriatr. 2023. PMID: 38093184 Free PMC article.
References
-
- Laliberté M-C, Normandeau M, Lord A, et al. . Use of over-the-counter medications and natural products in patients with moderate and severe chronic renal insufficiency. Am J Kidney Dis. 2007;49(2):245-56. - PubMed
-
- Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: part I. Definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician. 2004;70(5):869-76. - PubMed
-
- Levey AS, Eckardt KU, Tsukamoto Y, et al. . Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089-100. - PubMed
-
- Lalonde L, Normandeau M, Lamarre D, et al. . Evaluation of a training and communication-network nephrology program for community pharmacists. Pharm World Sci. 2008;30(6):924-33. - PubMed
-
- Manley HJ, Cannella CA, Bailie GR, St Peter WL. Medication-related problems in ambulatory hemodialysis patients: a pooled analysis. Am J Kidney Dis. 2005;46(4):669-80. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical