False-positive HIV diagnoses: lessons from Ugandan and Russian research cohorts
- PMID: 29384717
- PMCID: PMC5949866
- DOI: 10.1080/15284336.2018.1429846
False-positive HIV diagnoses: lessons from Ugandan and Russian research cohorts
Abstract
Background: Research studies rely on accurate assessment of entry criteria in order to maintain study integrity and participant safety, however, challenges can exist with HIV studies in international settings.
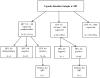
Objective: Examine the unexpectedly high proportion of study participants with an undetectable HIV viral load found in Ugandan and Russian research cohorts meeting antiretroviral therapy (ART)-naïve entry criteria.
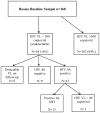
Methods: Russian participants with documented HIV and ART-naïve status were recruited between 2012 and 2015 from clinical and non-clinical sites in St. Petersburg. Participants in Uganda were recruited from Mbarara Regional Referral Hospital from 2011 to 2014 with documented HIV infection via rapid diagnostic testing and recorded ART-naïve in the clinic database. HIV viral load testing of baseline samples was performed; the lower limit of detection was 500 copies/mL in Russia and 40 in Uganda. Due to an unexpectedly high proportion of participants with undetectable viremia, additional tests were performed: enzyme-linked immunosorbent assay HIV testing and testing for ART.
Results: In Russia, 16% (58/360) had undetectable viremia; 3% (9/360) re-tested HIV-seronegative and 4% (13/360) tested positive for ART. In Uganda 11% (55/482) had undetectable viremia; 5% (26/482) re-tested HIV-seronegative, while <1% (4/482) tested positive for ART.
Conclusions: In both Russia & Uganda, undetectable viremia was much higher than would be expected for an HIV-infected ART-naïve cohort. Misclassification of study participants was due to misdiagnosis of HIV with rapid diagnostic testing and inaccurate accounting of ART use. Confirmatory HIV testing could improve accuracy of participants meeting entry criteria for HIV infection as might increased scrutiny of medication use in an ART-naïve cohort.
Keywords: Diagnostic screening; HIV/AIDS; Rapid diagnostic testing; Russia; Uganda.
Conflict of interest statement
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism(NIAAA) (K24AA022586, U01AA020780, U24AA020779, U24AA020778, U01AA020776 and U01AA021989) and the National Institute for Allergy and Infectious Diseases (NIAID) Center for AIDS Research (CFAR) (P30 AI027763, P30AI042853). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or other funding agencies. The authors report no declarations of interest.
Figures
Similar articles
-
Prevalence of undetectable and suppressed viral load in HIV-infected pregnant women initiating Option B+ in Uganda: an observational study nested within a randomized controlled trial.BMC Infect Dis. 2021 Sep 4;21(1):907. doi: 10.1186/s12879-021-06608-4. BMC Infect Dis. 2021. PMID: 34481464 Free PMC article. Clinical Trial.
-
Short Communication: Validation of the Asante HIV-1 Rapid Recency Assay for Detection of Recent HIV-1 Infections in Uganda.AIDS Res Hum Retroviruses. 2021 Dec;37(12):893-896. doi: 10.1089/AID.2020.0279. Epub 2021 Feb 25. AIDS Res Hum Retroviruses. 2021. PMID: 33499732 Free PMC article.
-
Population prevalence of antiretroviral therapy sharing and its association with HIV viremia in rural Uganda: a cross-sectional population-based study.J Int AIDS Soc. 2023 Sep;26(9):e26135. doi: 10.1002/jia2.26135. J Int AIDS Soc. 2023. PMID: 37705364 Free PMC article.
-
HIV misdiagnosis in sub-Saharan Africa: performance of diagnostic algorithms at six testing sites.J Int AIDS Soc. 2017 Jul 3;20(1):21419. doi: 10.7448/IAS.20.1.21419. J Int AIDS Soc. 2017. PMID: 28691437 Free PMC article. Review.
-
[Consensus document of Gesida and Spanish Secretariat for the National Plan on AIDS (SPNS) regarding combined antiretroviral treatment in adults infected by the human immunodeficiency virus (January 2012)].Enferm Infecc Microbiol Clin. 2012 Jun;30(6):e1-89. doi: 10.1016/j.eimc.2012.03.006. Epub 2012 May 23. Enferm Infecc Microbiol Clin. 2012. PMID: 22633764 Spanish.
Cited by
-
Optimizing HIV testing services in sub-Saharan Africa: cost and performance of verification testing with HIV self-tests and tests for triage.J Int AIDS Soc. 2019 Mar;22 Suppl 1(Suppl Suppl 1):e25237. doi: 10.1002/jia2.25237. J Int AIDS Soc. 2019. PMID: 30907507 Free PMC article.
-
High level of HIV false positives using EIA-based algorithm in survey: Importance of confirmatory testing.PLoS One. 2020 Oct 22;15(10):e0239782. doi: 10.1371/journal.pone.0239782. eCollection 2020. PLoS One. 2020. PMID: 33091019 Free PMC article.
-
Sensitivity and specificity of the new Bio-Rad HIV screening test, Access HIV combo V2.J Clin Microbiol. 2024 May 8;62(5):e0009524. doi: 10.1128/jcm.00095-24. Epub 2024 Mar 27. J Clin Microbiol. 2024. PMID: 38534108 Free PMC article.
-
Association between alcohol use and inflammatory biomarkers over time among younger adults with HIV-The Russia ARCH Observational Study.PLoS One. 2019 Aug 22;14(8):e0219710. doi: 10.1371/journal.pone.0219710. eCollection 2019. PLoS One. 2019. PMID: 31437155 Free PMC article.
-
Challenges faced by the HIV testing system in low- and middle-income countries.Afr J Lab Med. 2023 Jan 31;12(1):1974. doi: 10.4102/ajlm.v12i1.1974. eCollection 2023. Afr J Lab Med. 2023. PMID: 36756215 Free PMC article.
References
-
- Center for Disease Control and Prevention. [Accessed July 14, 2017];Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. 2014 https://stacks.cdc.gov/view/cdc/23447.
-
- Johnson C, Fonner V, Sands A, et al. Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection. Geneva: World Health Organization; 2015. A report on the misdiagnosis of HIV status. Vol ANNEX 14. - PubMed
-
- Klarkowski D, O’Brien DP, Shanks L, Singh KP. Causes of false-positive HIV rapid diagnostic test results. Expert Rev Anti Infect Ther. 2014;12(1):49–62. - PubMed
-
- Parisi MR, Soldini L, Di Perri G, Tiberi S, Lazzarin A, Lillo FB. Offer of rapid testing and alternative biological samples as practical tools to implement HIV screening programs. New Microbiol. 2009;32(4):391–396. - PubMed
-
- De Cock KM, Bunnell R, Mermin J. Unfinished business--expanding HIV testing in developing countries. N Engl J Med. 2006;354(5):440–442. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
