Meeting the Challenge: The National Cancer Institute's Central Institutional Review Board for Multi-Site Research
- PMID: 29384720
- PMCID: PMC5844669
- DOI: 10.1200/JCO.2017.76.9836
Meeting the Challenge: The National Cancer Institute's Central Institutional Review Board for Multi-Site Research
Abstract
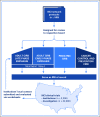
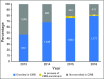
The National Institutes of Health (NIH) issued a new policy that requires a single institutional review board (IRB) of record be used for all protocols funded by the NIH that are carried out at more than one site in the United States, effective January 2018. This policy affects several hundred clinical trials opened annually across the NIH. Limited data exist to compare the use of a single IRB to that of multiple local IRBs, so some institutions are resistant to or distrustful of single IRBs. Since 2001, the National Cancer Institute (NCI) has funded a central IRB (CIRB) that provides human patient reviews for its extensive national cancer clinical trials program. This paper presents data to show the adoption, efficiencies gained, and satisfaction of the CIRB among NCI trial networks and reviews key lessons gleaned from 16 years of experience that may be informative for others charged with implementation of the new NIH single-IRB policy.
Figures
References
-
- The Lancet Neurology : NeuroNEXT: accelerating drug development in neurology. Lancet Neurol 11:119, 2012 - PubMed
-
- National Conference on Alternative IRB Models: Optimizing human subject protection, 2006:1-53. (2017). In: National conference on alternative IRB models: Optimizing human subject protection. https://www.aamc.org/download/75240/data/irbconf06rpt.pdf.
-
- Pyle S.: Benefits of working with a central IRB. Improved efficiencies and enhanced human subject protections. Monitor (Charlottet):9-12, 2013
-
- Weschler J: Commentary- View from Washington-Central vs. Local: Rethinking IRBs-regulators and sponsors encourage alternative review models to fit a growing research enterprise. Appl Clin Trials 16:24-26, 2007
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources