Efficacy and safety of micafungin for the treatment of patients with proven or probable invasive aspergillosis: A non-comparative, multicenter, phase IV, open-label study
- PMID: 29384927
- PMCID: PMC6392632
- DOI: 10.1097/MD.0000000000009443
Efficacy and safety of micafungin for the treatment of patients with proven or probable invasive aspergillosis: A non-comparative, multicenter, phase IV, open-label study
Abstract
Introduction: Few studies have assessed the efficacy and safety of micafungin in patients with proven or probable invasive aspergillosis (IA). This was the aim of the current study, which was conducted in 22 hospitals in China, where micafungin was approved for treatment of IA in 2006.
Methods: This was a non-comparative, phase IV open-label study (NCT02646774). Eligible patient were adults with proven or probable IA. Efficacy endpoints included rates of overall treatment success (primary endpoint) and clinical improvement, fungal clearance, mortality, and the site of Aspergillus infection (all secondary endpoints). Safety endpoints included incidences of treatment-emergent adverse events (TEAEs), serious AEs (SAEs), and adverse drug reactions (ADRs). These endpoints were reported descriptively with associated 95% confidence intervals (CI); no hypotheses were tested.
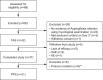
Results: The study was discontinued early due to low patient recruitment, which did not allow for the planned sample size to be reached. In total, 68 patients were enrolled: 42 into the full analysis set (for efficacy) and 61 into the safety analysis set. All patients were Han Chinese; the majority were male (n = 26; 61.9%) and ≤60 years of age (n = 35; 83.3%). Rates of overall treatment success, clinical improvement, fungal clearance, and mortality were 45.2% (n = 19/42; 95% CI: 29.85-61.33); 59.5% (n = 25/42; 95% CI: 43.28-74.37), 80.0% (n = 4/5; 95% CI: 28.36-99.49), and 7.1% (n = 3/42; 95% CI: 1.50-19.48), respectively. All patients were diagnosed with pulmonary Aspergillus infection. Overall, 155 TEAEs and 8 SAEs were reported by 37 (60.7%) and 7 (11.5%) patients. The most common TEAEs were decreased platelet count and fatigue (both n = 5; 8.2%) and the most common SAEs were intracranial hemorrhage and lung infection (n = 3; 4.9% and n = 2; 3.3%). Eight ADRs (n = 6; 9.8%) were reported but all were completely remitted or remitting during follow-up.
Conclusions: Results suggest that micafungin is efficacious and well-tolerated in patients with proven or probable IA in China. However, these findings should be interpreted with care, due to the small number of patients included in this study. Further comparative trials should be used to confirm the efficacy and safety of micafungin in patients with proven or probable IA.
Copyright © 2017 The Authors. Published by Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
Similar articles
-
Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis.J Infect. 2006 Nov;53(5):337-49. doi: 10.1016/j.jinf.2006.03.003. Epub 2006 May 6. J Infect. 2006. PMID: 16678903 Free PMC article. Clinical Trial.
-
Multicenter, randomized, open-label study comparing the efficacy and safety of micafungin versus itraconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplant.Biol Blood Marrow Transplant. 2012 Oct;18(10):1509-16. doi: 10.1016/j.bbmt.2012.03.014. Epub 2012 Mar 30. Biol Blood Marrow Transplant. 2012. PMID: 22469884 Clinical Trial.
-
Efficacy and safety of micafungin as an empirical therapy for invasive fungal infections in patients with hematologic disorders: a multicenter, prospective study.Ann Hematol. 2011 Oct;90(10):1209-17. doi: 10.1007/s00277-011-1277-1. Epub 2011 Jun 22. Ann Hematol. 2011. PMID: 21695388 Clinical Trial.
-
Micafungin for the treatment of invasive aspergillosis.J Infect. 2014 Jun;68(6):507-26. doi: 10.1016/j.jinf.2014.01.007. Epub 2014 Jan 27. J Infect. 2014. PMID: 24480373 Review.
-
[Micafungin: experimental therapy of fungal infections in animal models].Rev Iberoam Micol. 2009 Mar 31;26(1):42-8. doi: 10.1016/S1130-1406(09)70007-5. Epub 2009 May 7. Rev Iberoam Micol. 2009. PMID: 19463276 Review. Spanish.
Cited by
-
Coagulation dysfunction events associated with echinocandins: a real-world study from FDA adverse event reporting system (FAERS) database.Thromb J. 2024 Aug 23;22(1):78. doi: 10.1186/s12959-024-00641-4. Thromb J. 2024. PMID: 39180077 Free PMC article.
-
Pulmonary aspergilloma coexisting with hamartoma in post pulmonary tuberculosis: A case report.Respir Med Case Rep. 2022 Sep 16;39:101738. doi: 10.1016/j.rmcr.2022.101738. eCollection 2022. Respir Med Case Rep. 2022. PMID: 36164491 Free PMC article.
-
Comparative Pharmacodynamics of Echinocandins against Aspergillus fumigatus Using an In Vitro Pharmacokinetic/Pharmacodynamic Model That Correlates with Clinical Response to Caspofungin Therapy: Is There a Place for Dose Optimization?Antimicrob Agents Chemother. 2021 Mar 18;65(4):e01618-20. doi: 10.1128/AAC.01618-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33495222 Free PMC article.
-
Distribution of Aspergillus species and risk factors for aspergillosis in mainland China: a systematic review.Ther Adv Infect Dis. 2024 Jun 4;11:20499361241252537. doi: 10.1177/20499361241252537. eCollection 2024 Jan-Dec. Ther Adv Infect Dis. 2024. PMID: 38835831 Free PMC article. Review.
References
-
- Pagano L, Girmenia C, Mele L, et al. Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA Infection Program. Haematologica 2001;86:862–70. - PubMed
-
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813–21. - PMC - PubMed
-
- Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 2003;102:827–33. - PubMed
-
- Pagano L, Caira M, Picardi M, et al. Invasive Aspergillosis in patients with acute leukemia: update on morbidity and mortality–SEIFEM-C Report. Clin Infect Dis 2007;44:1524–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

