Implementation of Glycan Remodeling to Plant-Made Therapeutic Antibodies
- PMID: 29385073
- PMCID: PMC5855643
- DOI: 10.3390/ijms19020421
Implementation of Glycan Remodeling to Plant-Made Therapeutic Antibodies
Abstract
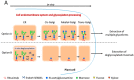
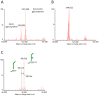
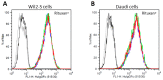
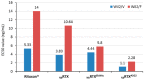
N-glycosylation profoundly affects the biological stability and function of therapeutic proteins, which explains the recent interest in glycoengineering technologies as methods to develop biobetter therapeutics. In current manufacturing processes, N-glycosylation is host-specific and remains difficult to control in a production environment that changes with scale and production batches leading to glycosylation heterogeneity and inconsistency. On the other hand, in vitro chemoenzymatic glycan remodeling has been successful in producing homogeneous pre-defined protein glycoforms, but needs to be combined with a cost-effective and scalable production method. An efficient chemoenzymatic glycan remodeling technology using a plant expression system that combines in vivo deglycosylation with an in vitro chemoenzymatic glycosylation is described. Using the monoclonal antibody rituximab as a model therapeutic protein, a uniform Gal2GlcNAc2Man3GlcNAc2 (A2G2) glycoform without α-1,6-fucose, plant-specific α-1,3-fucose or β-1,2-xylose residues was produced. When compared with the innovator product Rituxan®, the plant-made remodeled afucosylated antibody showed similar binding affinity to the CD20 antigen but significantly enhanced cell cytotoxicity in vitro. Using a scalable plant expression system and reducing the in vitro deglycosylation burden creates the potential to eliminate glycan heterogeneity and provide affordable customization of therapeutics' glycosylation for maximal and targeted biological activity. This feature can reduce cost and provide an affordable platform to manufacture biobetter antibodies.
Keywords: N-glycosylation; Nicotiana benthamiana; glycan remodeling; recombinant glycoproteins; therapeutic proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

