Advances in the Development of PET Ligands Targeting Histone Deacetylases for the Assessment of Neurodegenerative Diseases
- PMID: 29385079
- PMCID: PMC6017260
- DOI: 10.3390/molecules23020300
Advances in the Development of PET Ligands Targeting Histone Deacetylases for the Assessment of Neurodegenerative Diseases
Abstract
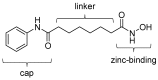
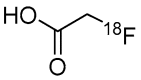
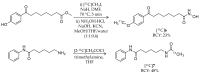
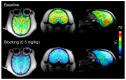
Epigenetic alterations of gene expression have emerged as a key factor in several neurodegenerative diseases. In particular, inhibitors targeting histone deacetylases (HDACs), which are enzymes responsible for deacetylation of histones and other proteins, show therapeutic effects in animal neurodegenerative disease models. However, the details of the interaction between changes in HDAC levels in the brain and disease progression remain unknown. In this review, we focus on recent advances in development of radioligands for HDAC imaging in the brain with positron emission tomography (PET). We summarize the results of radiosynthesis and biological evaluation of the HDAC ligands to identify their successful results and challenges. Since 2006, several small molecules that are radiolabeled with a radioisotope such as carbon-11 or fluorine-18 have been developed and evaluated using various assays including in vitro HDAC binding assays and PET imaging in rodents and non-human primates. Although most compounds do not readily cross the blood-brain barrier, adamantane-conjugated radioligands tend to show good brain uptake. Until now, only one HDAC radioligand has been tested clinically in a brain PET study. Further PET imaging studies to clarify age-related and disease-related changes in HDACs in disease models and humans will increase our understanding of the roles of HDACs in neurodegenerative diseases.
Keywords: histone deacetylase; imaging; neurodegenerative disease; positron emission tomography; radioligand.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Radionuclide labeling and evaluation of candidate radioligands for PET imaging of histone deacetylase in the brain.Bioorg Med Chem Lett. 2013 Dec 15;23(24):6700-5. doi: 10.1016/j.bmcl.2013.10.038. Epub 2013 Oct 30. Bioorg Med Chem Lett. 2013. PMID: 24210501 Free PMC article.
-
Preclinical validation of a novel brain-penetrant PET ligand for visualization of histone deacetylase 6: a potential imaging target for neurodegenerative diseases.Eur J Nucl Med Mol Imaging. 2024 Jul;51(8):2193-2203. doi: 10.1007/s00259-024-06666-1. Epub 2024 Mar 5. Eur J Nucl Med Mol Imaging. 2024. PMID: 38441662
-
HDAC Inhibitors: Innovative Strategies for Their Design and Applications.Molecules. 2022 Jan 21;27(3):715. doi: 10.3390/molecules27030715. Molecules. 2022. PMID: 35163980 Free PMC article. Review.
-
Evaluation of Class IIa Histone Deacetylases Expression and In Vivo Epigenetic Imaging in a Transgenic Mouse Model of Alzheimer's Disease.Int J Mol Sci. 2021 Aug 11;22(16):8633. doi: 10.3390/ijms22168633. Int J Mol Sci. 2021. PMID: 34445342 Free PMC article.
-
Targeting histone deacetylases for heart diseases.Bioorg Chem. 2023 Sep;138:106601. doi: 10.1016/j.bioorg.2023.106601. Epub 2023 May 10. Bioorg Chem. 2023. PMID: 37224740 Review.
Cited by
-
The role of neuroimaging in Parkinson's disease.J Neurochem. 2021 Nov;159(4):660-689. doi: 10.1111/jnc.15516. Epub 2021 Oct 3. J Neurochem. 2021. PMID: 34532856 Free PMC article. Review.
-
Substance use disorders: a comprehensive update of classification, epidemiology, neurobiology, clinical aspects, treatment and prevention.World Psychiatry. 2023 Jun;22(2):203-229. doi: 10.1002/wps.21073. World Psychiatry. 2023. PMID: 37159360 Free PMC article.
-
Advances in PET/CT Imaging for Breast Cancer.J Clin Med. 2023 Jul 7;12(13):4537. doi: 10.3390/jcm12134537. J Clin Med. 2023. PMID: 37445572 Free PMC article. Review.
-
Elaboration of the Effective Multi-Target Therapeutic Platform for the Treatment of Alzheimer's Disease Based on Novel Monoterpene-Derived Hydroxamic Acids.Int J Mol Sci. 2023 Jun 4;24(11):9743. doi: 10.3390/ijms24119743. Int J Mol Sci. 2023. PMID: 37298694 Free PMC article.
-
The Anti-Inflammatory Effect of Preventive Intervention with Ketogenic Diet Mediated by the Histone Acetylation of mGluR5 Promotor Region in Rat Parkinson's Disease Model: A Dual-Tracer PET Study.Parkinsons Dis. 2022 Sep 5;2022:3506213. doi: 10.1155/2022/3506213. eCollection 2022. Parkinsons Dis. 2022. PMID: 36105302 Free PMC article.
References
-
- Lovrečić L., Maver A., Zadel M., Peterlin B. Neurodegenerative Diseases. InTech; London, UK: 2013. The Role of Epigenetics in Neurodegenerative Diseases; pp. 345–365.
-
- Turek-Plewa J., Jagodzinski P.P. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell. Mol. Biol. Lett. 2005;10:631–647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

