Faecalibacterium prausnitzii and a Prebiotic Protect Intestinal Health in a Mouse Model of Antibiotic and Clostridium difficile Exposure
- PMID: 29385239
- PMCID: PMC6068000
- DOI: 10.1002/jpen.1053
Faecalibacterium prausnitzii and a Prebiotic Protect Intestinal Health in a Mouse Model of Antibiotic and Clostridium difficile Exposure
Abstract
Background: Clostridium difficile (CD) infection (CDI) increases patient morbidity, mortality and healthcare costs. Antibiotic treatment induces gut dysbiosis and is both a major risk factor for CD colonization and treatment of CDI. Probiotics have been trialed to support commensal gut microbiota and reduce CDI. This study investigated commensal microbe Faecalibacterium prausnitzii (FP) and a prebiotic, both known to yield butyrate and be anti-inflammatory and immunomodulatory, on CD colonization and gut integrity in mice.
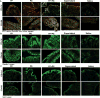
Methods: Mice were randomly grouped and supplemented daily with FP, prebiotic, FP + prebiotic, FP/prebiotic supernatant, or saline throughout the entire study. Following treatment with clindamycin for 3 days, mice were exposed to CD. Feces were collected at baseline, the day after antibiotic, and 1, 3, and 5 days after CD exposure and cultured for bacterial overgrowth and CD colonization. On days 1 and 5 after CD exposure, mice were randomly euthanized, and proximal colon was dissected for histological analysis and preparation of RNA for analysis of proinflammatory and anti-inflammatory cytokines.
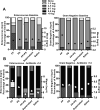
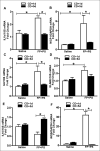
Results: Although all mice exhibited bacterial overgrowth and CD colonization, bacterial burden resolved quicker in the FP + prebiotic group. This was associated with induction and resolution of innate immune responses, anion exchanger, and tight junction protein preservation in proximal colon. CD toxin virulence potential was questionable as expression of CD toxin B receptor was depleted in the FP + prebiotic group.
Conclusion: Supplementation with anti-inflammatory butyrate-supporting commensal bacteria and prebiotic may support innate immune responses and minimize bacterial burden and negative effects during antibiotic and CD exposure.
Keywords: antibiotics; butyrate, Clostridium difficile, innate immunity, intestine, microbiome, prebiotic, probiotics.
© 2018 American Society for Parenteral and Enteral Nutrition.
Conflict of interest statement
Conflicts of interest: None declared. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures

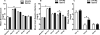

References
-
- Sehulster L, Chinn RYW. Guidelines for environmental infection control in health-care facilities. MMWR Recomm Rep. 2003;52(RR10):1–42. Available from: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_excerpt.html. - PubMed
-
- Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and Bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. - PubMed
-
- Backhed F, Ley R, Sonnenburg J, Peterson D, Gordon J. Hostbacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

