Toxicity implications for early life stage Japanese medaka (Oryzias latipes) exposed to oxyfluorfen
- PMID: 29385312
- PMCID: PMC5912988
- DOI: 10.1002/tox.22541
Toxicity implications for early life stage Japanese medaka (Oryzias latipes) exposed to oxyfluorfen
Abstract
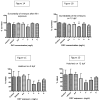
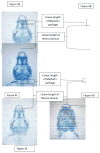
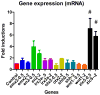
We investigated the potential toxic effects of Oxyfluorfen (OXY), an herbicide used in agriculture, on the embryo-larval development of Japanese medaka fish (Oryzias latipes). Embryos (1-day postfertilization) and larvae (2-day posthatch) were exposed to OXY (0.5-8 mg/L) for 96 h and evaluated for mortality and hatching on embryos, and the mortality and growth on larvae during depuration. It was observed that the embryo-mortality was inconsistently altered by OXY; only the 2 mg/L group showed significant reduction on embryo survivability. However, larval-mortality was concentration-dependent and OXY exposure induced scoliosis-like phenotypic features in the surviving larvae; the calculated LC50 was 5.238 mg/L. Our data further indicated that larval skeleton, both axial and appendicular, was the potential target site of OXY. Skeletal growth in larvae exposed to 2 mg/L was inhibited significantly until 1 week of depuration with regard to the linear lengths of neurocranium, Meckel's cartilage, caudal vertebrae (first 10) in the axial skeletons, and pectoral fin and urostyle in the appendicular skeletons. Moreover, the total protein content remained unaltered by OXY after 96 h exposure; while the RNA concentration was reduced significantly in larvae exposed to 2 mg/L. Expression analysis of several genes by quantitative real-time RT-PCR (RT-qPCR) showed significant upregulation of zic5, a zinc-finger type transcription regulator, at the transcription level. This study indicated that the scoliosis induced by OXY in Japanese medaka larvae was the result of stunted skeletal growth, probably because of deregulation of zinc-finger type transcription regulators, at the genomic level.
Keywords: Japanese medaka; developmental toxicity; gene expression; oxyfluorfen; skeleton.
© 2018 Wiley Periodicals, Inc.
Figures









Similar articles
-
Developmental and epigenetic effects of Roundup and glyphosate exposure on Japanese medaka (Oryzias latipes).Aquat Toxicol. 2019 May;210:215-226. doi: 10.1016/j.aquatox.2019.03.005. Epub 2019 Mar 7. Aquat Toxicol. 2019. PMID: 30875550
-
Assessing the toxicity of sediments using the medaka embryo-larval assay and 2 other bioassays.Environ Toxicol Chem. 2016 Sep;35(9):2270-80. doi: 10.1002/etc.3388. Epub 2016 Jun 15. Environ Toxicol Chem. 2016. PMID: 26823140
-
Stage-dependent differences in effects of carbaryl on population growth rate in Japanese medaka (Oryzias latipes).Environ Toxicol Chem. 2008 Nov;27(11):2397-402. doi: 10.1897/08-073.1. Environ Toxicol Chem. 2008. PMID: 18498201
-
Life-cycle experiments of medaka fish aboard the international space station.Adv Space Biol Med. 2003;9:201-16. doi: 10.1016/s1569-2574(03)09008-7. Adv Space Biol Med. 2003. PMID: 14631634 Review.
-
A Baseline for Skeletal Investigations in Medaka (Oryzias latipes): The Effects of Rearing Density on the Postcranial Phenotype.Front Endocrinol (Lausanne). 2022 Jun 30;13:893699. doi: 10.3389/fendo.2022.893699. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35846331 Free PMC article. Review.
Cited by
-
A systematic review of the evaluation of endocrine-disrupting chemicals in the Japanese medaka (Oryzias latipes) fish.Front Toxicol. 2023 Nov 27;5:1272368. doi: 10.3389/ftox.2023.1272368. eCollection 2023. Front Toxicol. 2023. PMID: 38090358 Free PMC article.
-
Molecular mechanisms of environmental pollutant-induced cartilage damage: from developmental disorders to osteoarthritis.Arch Toxicol. 2024 Sep;98(9):2763-2796. doi: 10.1007/s00204-024-03772-9. Epub 2024 May 17. Arch Toxicol. 2024. PMID: 38758407 Review.
References
-
- Narayanan KB, Ali M, Barclay BJ, Cheng Q, D’Abronzo L, Dornetshuber-Fleiss R, Ghosh PM, Guzman MJG, Lee T-J, Leung PS, Li L, Luanpitpong S, Ratovitski E, Rojanasakul Y, Romano MF, Romano S, Sinha RK, Yedjou MF, Al-Mulla F, Al-Temaimi R, Amedei A, Brown DG, Ryan EP, Colacci AM, Hamid RA, Mondello C, Raju J, Salem HK, Woodrick J, Scovassi AI, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Kim SY, Bisson WH, Lowe L, Park HH. Disruptive environmental chemicals and cellular mechanisms that confer resistance to cell death. Carcinogenesis. 2015;36:s89–s110. - PMC - PubMed
-
- U.S. EPA. Oxyfluorfen: Human health risk assessment. Health effects division (HED) chapter for registration eligibility decision (RED) document. Registration case no. 2490. Chemical no. 111601. 2002
-
- Matringe M, Camadro JM, Labbe P, Scalla R. Protoporphyrinogen oxidase inhibition by three per oxidizing herbicides: oxadiazon, LS 82–556 and M&B 39279. FEBS Letters. 1989a;245:35–38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

