Understanding Signal and Background in a Thermally Resolved, Single-Branched DNA Assay Using Square Wave Voltammetry
- PMID: 29385341
- PMCID: PMC6008162
- DOI: 10.1021/acs.analchem.8b00036
Understanding Signal and Background in a Thermally Resolved, Single-Branched DNA Assay Using Square Wave Voltammetry
Abstract
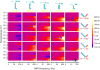
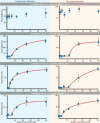
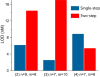
Electrochemical bioanalytical sensors with oligonucleotide transducer molecules have been recently extended for quantifying a wide range of biomolecules, from small drugs to large proteins. Short DNA or RNA strands have gained attention recently due to the existence of circulating oligonucleotides in human blood, yet challenges remain for adequately sensing these targets at electrode surfaces. In this work, we have developed a quantitative electrochemical method which uses target-induced proximity of a single-branched DNA structure to drive hybridization at an electrode surface, with readout by square-wave voltammetry (SWV). Using custom instrumentation, we first show that precise control of temperature can provide both electrochemical signal amplification and background signal depreciation in SWV readout of small oligonucleotides. Next, we thoroughly compared 25 different combinations of binding energies by their signal-to-background ratios and differences. These data served as a guide to select the optimal parameters of binding energy, SWV frequency, and assay temperature. Finally, the influence of experimental workflow on the sensitivity and limit of detection (LOD) of the sensor is demonstrated. This study highlights the importance of precisely controlling temperature and SWV frequency in DNA-driven assays on electrode surfaces while also presenting a novel instrumental design for fine-tuning of such systems.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Characterization of an electrochemical mercury sensor using alternating current, cyclic, square wave and differential pulse voltammetry.Anal Chim Acta. 2014 Jan 31;810:79-85. doi: 10.1016/j.aca.2013.12.005. Epub 2013 Dec 11. Anal Chim Acta. 2014. PMID: 24439508
-
Electrochemical branched-DNA assay for polymerase chain reaction-free detection and quantification of oncogenes in messenger RNA.Anal Chem. 2008 Dec 15;80(24):9402-10. doi: 10.1021/ac801263r. Anal Chem. 2008. PMID: 19007191
-
Effect of nanoporous gold thin film morphology on electrochemical DNA sensing.Anal Chem. 2015 Aug 18;87(16):8149-56. doi: 10.1021/acs.analchem.5b00846. Epub 2015 Apr 30. Anal Chem. 2015. PMID: 25892217
-
Voltammetric behaviour of free DNA bases, methylcytosine and oligonucleotides at disposable screen printed graphite electrode platforms.Analyst. 2013 Sep 21;138(18):5239-49. doi: 10.1039/c3an00682d. Epub 2013 Jul 16. Analyst. 2013. PMID: 23857474
-
Fabrication strategies, sensing modes and analytical applications of ratiometric electrochemical biosensors.Biosens Bioelectron. 2017 May 15;91:523-537. doi: 10.1016/j.bios.2017.01.011. Epub 2017 Jan 7. Biosens Bioelectron. 2017. PMID: 28086123 Review.
Cited by
-
Ligand-Based Stability Changes in Duplex DNA Measured with a Microscale Electrochemical Platform.Biosensors (Basel). 2019 Apr 12;9(2):54. doi: 10.3390/bios9020054. Biosensors (Basel). 2019. PMID: 31013753 Free PMC article.
-
Nucleic-Acid Driven Cooperative Bioassays Using Probe Proximity or Split-Probe Techniques.Anal Chem. 2021 Jan 12;93(1):198-214. doi: 10.1021/acs.analchem.0c04364. Epub 2020 Nov 4. Anal Chem. 2021. PMID: 33147015 Free PMC article. Review.
-
Effects of Nucleic Acid Structural Heterogeneity on the Electrochemistry of Tethered Redox Molecules.Langmuir. 2022 Jun 14;38(23):7322-7330. doi: 10.1021/acs.langmuir.2c00840. Epub 2022 May 31. Langmuir. 2022. PMID: 35639972 Free PMC article.
-
Nonfaradaic Current Suppression in DNA-Based Electrochemical Assays with a Differential Potentiostat.Anal Chem. 2019 Dec 17;91(24):15833-15839. doi: 10.1021/acs.analchem.9b04149. Epub 2019 Dec 3. Anal Chem. 2019. PMID: 31718147 Free PMC article.
-
A Nucleic Acid Nanostructure Built through On-Electrode Ligation for Electrochemical Detection of a Broad Range of Analytes.J Am Chem Soc. 2019 Jul 24;141(29):11721-11726. doi: 10.1021/jacs.9b06229. Epub 2019 Jul 9. J Am Chem Soc. 2019. PMID: 31257869 Free PMC article.
References
-
- Flechsig G-U, Peter J, Hartwich G, Wang J, Grüdler P. Langmuir. 2005;21(17):7848–7853. - PubMed
-
- Shen Z, Sintim HO, Semancik S. Anal. Chim. Acta. 2015;853:265–270. - PubMed
-
- Peterlinz KA, Georgiadis RM, et al. J. Am. Chem. Soc. 1997;119(14):3401–3402.
-
- Shen Z, Nakayama S, Semancik S, Sintim HO. Chem. Commun. 2012;48(61):7580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

