Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia
- PMID: 29385370
- PMCID: PMC5996391
- DOI: 10.1056/NEJMoa1709866
Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia
Abstract
Background: In a single-center phase 1-2a study, the anti-CD19 chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel produced high rates of complete remission and was associated with serious but mainly reversible toxic effects in children and young adults with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
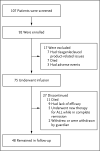
Methods: We conducted a phase 2, single-cohort, 25-center, global study of tisagenlecleucel in pediatric and young adult patients with CD19+ relapsed or refractory B-cell ALL. The primary end point was the overall remission rate (the rate of complete remission or complete remission with incomplete hematologic recovery) within 3 months.
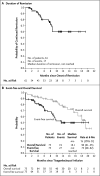
Results: For this planned analysis, 75 patients received an infusion of tisagenlecleucel and could be evaluated for efficacy. The overall remission rate within 3 months was 81%, with all patients who had a response to treatment found to be negative for minimal residual disease, as assessed by means of flow cytometry. The rates of event-free survival and overall survival were 73% (95% confidence interval [CI], 60 to 82) and 90% (95% CI, 81 to 95), respectively, at 6 months and 50% (95% CI, 35 to 64) and 76% (95% CI, 63 to 86) at 12 months. The median duration of remission was not reached. Persistence of tisagenlecleucel in the blood was observed for as long as 20 months. Grade 3 or 4 adverse events that were suspected to be related to tisagenlecleucel occurred in 73% of patients. The cytokine release syndrome occurred in 77% of patients, 48% of whom received tocilizumab. Neurologic events occurred in 40% of patients and were managed with supportive care, and no cerebral edema was reported.
Conclusions: In this global study of CAR T-cell therapy, a single infusion of tisagenlecleucel provided durable remission with long-term persistence in pediatric and young adult patients with relapsed or refractory B-cell ALL, with transient high-grade toxic effects. (Funded by Novartis Pharmaceuticals; ClinicalTrials.gov number, NCT02435849 .).
Figures
Comment in
-
Immunotherapy: CAR T cell therapy efficacious against B-ALL across age groups.Nat Rev Clin Oncol. 2018 Apr;15(4):199. doi: 10.1038/nrclinonc.2018.32. Epub 2018 Feb 20. Nat Rev Clin Oncol. 2018. PMID: 29459644 No abstract available.
References
-
- Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34(Suppl 15):3011. abstract.
-
- Grupp SA, Maude SL, Shaw PA, et al. Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019) Blood. 2015;126:681. abstract.
-
- Grupp SA, Laetsch TW, Buechner J, et al. Analysis of a global registration trial of the efficacy and safety of CTL019 in pediatric and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) Blood. 2016;128:221. abstract.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
