The Effect of Low-Dose Naltrexone on Medication in Inflammatory Bowel Disease: A Quasi Experimental Before-and-After Prescription Database Study
- PMID: 29385430
- PMCID: PMC5972567
- DOI: 10.1093/ecco-jcc/jjy008
The Effect of Low-Dose Naltrexone on Medication in Inflammatory Bowel Disease: A Quasi Experimental Before-and-After Prescription Database Study
Erratum in
-
Corrigendum: The Effect of Low-Dose Naltrexone on Medication in Inflammatory Bowel Disease: A Quasi Experimental Before-and-After Prescription Database Study.J Crohns Colitis. 2019 Dec 10;13(12):1588-1589. doi: 10.1093/ecco-jcc/jjz101. J Crohns Colitis. 2019. PMID: 31499520 Free PMC article. No abstract available.
Abstract
Background and aims: Low-dose naltrexone [LDN] is a controversial off-label treatment used by many Crohn's disease [CD] and ulcerative colitis [UC] patients. A small number of preliminary studies indicate that LDN might be beneficial in CD, but evidence is too scarce to demonstrate efficacy. We sought to examine whether initiation of LDN therapy by patients with inflammatory bowel disease [IBD] was followed by changes in dispensing of relevant medication.
Methods: We performed a quasi-experimental before-and-after study following a sudden increase in LDN use in the Norwegian population in 2013. IBD patients were identified from among all the patients who had at least one LDN prescription recorded in the Norwegian Prescription Database [NorPD] in 2013. Drug dispensing 2 years before and after the first LDN prescription was compared.
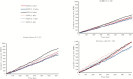
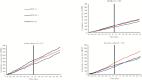
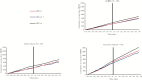
Results: We identified 582 IBD patients who had received LDN. Of the 256 patients who became persistent LDN users, there were reductions in the number of users for [i] all examined drugs [-12%], [ii] intestinal anti-inflammatory agents [-17%], [iii] other immunosuppressants [-29%], [iv] intestinal corticosteroids [-32%] and [v] aminosalicylates [-17%]. In subgroups of identified CD and UC patients, there were significant reductions in the number of users of intestinal corticosteroids [CD: -44%, UC: -53%] and systemic corticosteroids [UC: -24%]. No significant differences in cumulative defined daily doses were observed.
Conclusions: Our findings imply that the initiation of LDN in IBD is followed by reduced dispensing of several drugs considered essential in the treatment of CD and UC.
Figures
Similar articles
-
A sudden and unprecedented increase in low dose naltrexone (LDN) prescribing in Norway. Patient and prescriber characteristics, and dispense patterns. A drug utilization cohort study.Pharmacoepidemiol Drug Saf. 2017 Feb;26(2):136-142. doi: 10.1002/pds.4110. Epub 2016 Sep 26. Pharmacoepidemiol Drug Saf. 2017. PMID: 27670755 Free PMC article.
-
Low dose naltrexone: Effects on medication in rheumatoid and seropositive arthritis. A nationwide register-based controlled quasi-experimental before-after study.PLoS One. 2019 Feb 14;14(2):e0212460. doi: 10.1371/journal.pone.0212460. eCollection 2019. PLoS One. 2019. PMID: 30763385 Free PMC article.
-
Low-dose naltrexone and opioid consumption: a drug utilization cohort study based on data from the Norwegian prescription database.Pharmacoepidemiol Drug Saf. 2017 Jun;26(6):685-693. doi: 10.1002/pds.4201. Epub 2017 Mar 29. Pharmacoepidemiol Drug Saf. 2017. PMID: 28370746 Free PMC article.
-
Application of Drug Repurposing Approach for Therapeutic Intervention of Inflammatory Bowel Disease.Curr Rev Clin Exp Pharmacol. 2024;19(3):234-249. doi: 10.2174/0127724328245156231008154045. Curr Rev Clin Exp Pharmacol. 2024. PMID: 37859409 Review.
-
Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases.Aliment Pharmacol Ther. 2014 Aug;40(4):338-53. doi: 10.1111/apt.12838. Epub 2014 Jun 23. Aliment Pharmacol Ther. 2014. PMID: 24957164 Review.
Cited by
-
Low-dose naltrexone for the induction of remission in patients with mild to moderate Crohn's disease: protocol for the randomised, double-blinded, placebo-controlled, multicentre LDN Crohn study.BMJ Open. 2022 Apr 8;12(4):e058358. doi: 10.1136/bmjopen-2021-058358. BMJ Open. 2022. PMID: 35396307 Free PMC article.
-
Experimental Colitis Enhances the Rate of Antinociceptive Tolerance to Morphine via Peripheral Opioid Receptors.J Pharmacol Exp Ther. 2019 Sep;370(3):504-513. doi: 10.1124/jpet.119.256941. Epub 2019 Jun 27. J Pharmacol Exp Ther. 2019. PMID: 31248978 Free PMC article.
-
Changes in the consumption of antiepileptics and psychotropic medicines after starting low dose naltrexone: A nation-wide register-based controlled before-after study.Sci Rep. 2019 Oct 21;9(1):15085. doi: 10.1038/s41598-019-51569-z. Sci Rep. 2019. PMID: 31636347 Free PMC article.
-
Serious adverse events reported in placebo randomised controlled trials of oral naltrexone: a systematic review and meta-analysis.BMC Med. 2019 Jan 15;17(1):10. doi: 10.1186/s12916-018-1242-0. BMC Med. 2019. PMID: 30642329 Free PMC article.
-
Low-dose naltrexone's utility for non-cancer centralized pain conditions: a scoping review.Pain Med. 2023 Nov 2;24(11):1270-1281. doi: 10.1093/pm/pnad074. Pain Med. 2023. PMID: 37302106 Free PMC article.
References
-
- Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev 2010;12:CD001867. - PubMed
-
- CureTogether. 47 Crohn’s Disease Treatments Compared (Accessed January 2, 2018, at http://curetogether.com/crohns-disease/ig/treatment- effectiveness-vs-po...).
-
- CureTogether. 44 Ulcerative Colitis Treatments Compared (Accessed January, 2018, at http://curetogether.com/ulcerative-colitis/ig/treatment- effectiveness-v...).
-
- Smith JP, Stock H, Bingaman S, Mauger D, Rogosnitzky M, Zagon IS. Low-Dose Naltrexone Therapy Improves Active Crohn’s Disease. Am J Gastroenterol 2007;102:820–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

