Smooth muscle cell fate and plasticity in atherosclerosis
- PMID: 29385543
- PMCID: PMC5852505
- DOI: 10.1093/cvr/cvy022
Smooth muscle cell fate and plasticity in atherosclerosis
Erratum in
-
Corrigendum to: Smooth muscle cell fate and plasticity in atherosclerosis.Cardiovasc Res. 2018 Dec 1;114(14):1908. doi: 10.1093/cvr/cvy096. Cardiovasc Res. 2018. PMID: 29718279 Free PMC article. No abstract available.
Abstract
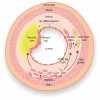
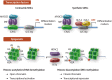
Current knowledge suggests that intimal smooth muscle cells (SMCs) in native atherosclerotic plaque derive mainly from the medial arterial layer. During this process, SMCs undergo complex structural and functional changes giving rise to a broad spectrum of phenotypes. Classically, intimal SMCs are described as dedifferentiated/synthetic SMCs, a phenotype characterized by reduced expression of contractile proteins. Intimal SMCs are considered to have a beneficial role by contributing to the fibrous cap and thereby stabilizing atherosclerotic plaque. However, intimal SMCs can lose their properties to such an extent that they become hard to identify, contribute significantly to the foam cell population, and acquire inflammatory-like cell features. This review highlights mechanisms of SMC plasticity in different stages of native atherosclerotic plaque formation, their potential for monoclonal or oligoclonal expansion, as well as recent findings demonstrating the underestimated deleterious role of SMCs in this disease.
Figures
References
-
- Dubland JA, Francis GA.. So much cholesterol: the unrecognized importance of smooth muscle cells in atherosclerotic foam cell formation . Curr Opin Lipidol 2016;27:155.. - PubMed
-
- Owens GK, Kumar MS, Wamhoff BR.. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004;84:767–801. - PubMed
-
- Orekhov AN, Andreeva ER, Mikhailova IA, Gordon D.. Cell proliferation in normal and atherosclerotic human aorta: proliferative splash in lipid-rich lesions. Atherosclerosis 1998;139:41–48. - PubMed
-
- Mulvihill ER, Jaeger J, Sengupta R, Ruzzo WL, Reimer C, Lukito S, Schwartz SM.. Atherosclerotic plaque smooth muscle cells have a distinct phenotype. Arterioscler Thromb Vasc Biol 2004;24:1283–1289. - PubMed
-
- Schwartz SM, deBlois D, O'Brien ER.. The intima. Soil for atherosclerosis and restenosis. Circ Res 1995;77:445–465. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

