Challenges Associated With Applying Physiologically Based Pharmacokinetic Modeling for Public Health Decision-Making
- PMID: 29385573
- PMCID: PMC6084449
- DOI: 10.1093/toxsci/kfy010
Challenges Associated With Applying Physiologically Based Pharmacokinetic Modeling for Public Health Decision-Making
Erratum in
-
Corrigendum to "Challenges Associated With Applying Physiologically Based Pharmacokinetic Modeling for Public Health Decision-Making".Toxicol Sci. 2018 Apr 1;162(2):724. doi: 10.1093/toxsci/kfy034. Toxicol Sci. 2018. PMID: 29528440 No abstract available.
Abstract
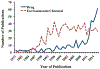
The development and application of physiologically based pharmacokinetic (PBPK) models in chemical toxicology have grown steadily since their emergence in the 1980s. However, critical evaluation of PBPK models to support public health decision-making across federal agencies has thus far occurred for only a few environmental chemicals. In order to encourage decision-makers to embrace the critical role of PBPK modeling in risk assessment, several important challenges require immediate attention from the modeling community. The objective of this contemporary review is to highlight 3 of these challenges, including: (1) difficulties in recruiting peer reviewers with appropriate modeling expertise and experience; (2) lack of confidence in PBPK models for which no tissue/plasma concentration data exist for model evaluation; and (3) lack of transferability across modeling platforms. Several recommendations for addressing these 3 issues are provided to initiate dialog among members of the PBPK modeling community, as these issues must be overcome for the field of PBPK modeling to advance and for PBPK models to be more routinely applied in support of public health decision-making.
Figures
References
-
- Agency for Toxic Substances and Disease Registry (ATSDR). (2016). Public Health Assessment for Corpus Christi Refineries (Site Wide Activities), Corpus Christi, Nueces County, Texas: Draft for Public Comment; August 29, 2016.
-
- Andersen ME, Black MB, Campbell JL, Pendse SN, Clewell III HJ, Pottenger LH, Bus JS, Dodd DE, Kemp DC, and McMullen PD (2017). Combining transcriptomics and PBPK modeling indicates a primary role of hypoxia and altered circadian signaling in dichloromethane carcinogenicity in mouse lung and liver. Toxicol. Appl. Pharmacol 332, 149–158. - PubMed
-
- Andersen ME, Clewell HJ III, Gargas ML, Smith FA, and Reitz RH (1987). Physiologically based pharmacokinetics and the risk assessment process for methylene chloride. Toxicol. Appl. Pharmacol 87, 185–205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

