Aging Exacerbates Neuroinflammatory Outcomes Induced by Acute Ozone Exposure
- PMID: 29385576
- PMCID: PMC5920500
- DOI: 10.1093/toxsci/kfy014
Aging Exacerbates Neuroinflammatory Outcomes Induced by Acute Ozone Exposure
Abstract
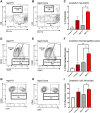
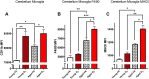
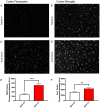
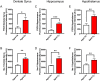
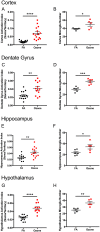
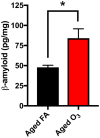
The role of environmental stressors, particularly exposure to air pollution, in the development of neurodegenerative disease remains underappreciated. We examined the neurological effects of acute ozone (O3) exposure in aged mice, where increased blood-brain barrier (BBB) permeability may confer vulnerability to neuroinflammatory outcomes. C57BL/6 male mice, aged 8-10 weeks or 12-18 months were exposed to either filtered air or 1.0 ppm O3 for 4 h; animals received a single IP injection of sodium fluorescein (FSCN) 20 h postexposure. One-hour post-FSCN injection, animals were transcardially perfused for immunohistochemical analysis of BBB permeability. β-amyloid protein expression was assessed via ELISA. Flow cytometric characterization of infiltrating immune cells, including neutrophils, macrophages, and microglia populations was performed 20 h post-O3 exposure. Flow cytometry analysis of brains revealed increased microglia "activation" and presentation of CD11b, F4/80, and MHCII in aged animals relative to younger ones; these age-induced differences were potentiated by acute O3 exposure. Cortical and limbic regions in aged brains had increased reactive microgliosis and β-amyloid protein expression after O3 insult. The aged cerebellum was particularly vulnerable to acute O3 exposure with increased populations of infiltrating neutrophils, peripheral macrophages/monocytes, and Ly6C+ inflammatory monocytes after insult, which were not significantly increased in the young cerebellum. O3 exposure increased the penetration of FSCN beyond the BBB, the infiltration of peripheral immune cells, and reactive gliosis of microglia. Thus, the aged BBB is vulnerable to insult and becomes highly penetrable in response to O3 exposure, leading to greater neuroinflammatory outcomes.
Figures




Similar articles
-
Mechanisms of response to ozone exposure: the role of mast cells in mice.Res Rep Health Eff Inst. 1999 Apr;(85):1-30; discussion 31-6. Res Rep Health Eff Inst. 1999. PMID: 10349676
-
Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice.Inhal Toxicol. 2016 Aug;28(9):383-92. doi: 10.1080/08958378.2016.1179373. Epub 2016 May 31. Inhal Toxicol. 2016. PMID: 27240593 Free PMC article.
-
Acute pulmonary effects of combined exposure to carbon nanotubes and ozone in mice.Inhal Toxicol. 2008 Feb;20(4):391-8. doi: 10.1080/08958370801904014. Inhal Toxicol. 2008. PMID: 18302047
-
Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications.J Toxicol Environ Health B Crit Rev. 1999 Jan-Mar;2(1):31-86. doi: 10.1080/109374099281232. J Toxicol Environ Health B Crit Rev. 1999. PMID: 10081525 Review.
-
Lung macrophages: current understanding of their roles in Ozone-induced lung diseases.Crit Rev Toxicol. 2020 Apr;50(4):310-323. doi: 10.1080/10408444.2020.1762537. Epub 2020 May 27. Crit Rev Toxicol. 2020. PMID: 32458707 Free PMC article. Review.
Cited by
-
Association between air pollution and CSF sTREM2 in cognitively normal older adults: The CABLE study.Ann Clin Transl Neurol. 2022 Nov;9(11):1752-1763. doi: 10.1002/acn3.51671. Epub 2022 Oct 31. Ann Clin Transl Neurol. 2022. PMID: 36317226 Free PMC article.
-
Induction of Accelerated Aging in a Mouse Model.Cells. 2022 Apr 22;11(9):1418. doi: 10.3390/cells11091418. Cells. 2022. PMID: 35563724 Free PMC article. Review.
-
Exposure to multiple ambient air pollutants changes white matter microstructure during early adolescence with sex-specific differences.Commun Med (Lond). 2024 Aug 1;4(1):155. doi: 10.1038/s43856-024-00576-x. Commun Med (Lond). 2024. PMID: 39090375 Free PMC article.
-
Air Pollution and Cognitive Impairment Among the Chinese Elderly Population: An Analysis of the Chinese Longitudinal Healthy Longevity Survey (CLHLS).Geohealth. 2025 Jan 6;9(1):e2024GH001023. doi: 10.1029/2024GH001023. eCollection 2025 Jan. Geohealth. 2025. PMID: 39776607 Free PMC article.
-
Neuroinflammatory and Neurometabolomic Consequences From Inhaled Wildfire Smoke-Derived Particulate Matter in the Western United States.Toxicol Sci. 2022 Feb 28;186(1):149-162. doi: 10.1093/toxsci/kfab147. Toxicol Sci. 2022. PMID: 34865172 Free PMC article.
References
-
- Ailshire J. A., Crimmins E. M. (2014). Fine particulate matter air pollution and cognitive function among older US adults. Am. J. Epidemiol. 180, 359–366.http://dx.doi.org/10.1093/aje/kwu155 - DOI - PMC - PubMed
-
- Aragon M. J., Topper L., Tyler C. R., Sanchez B., Zychowski K., Young T., Herbert G., Hall P., Erdely A., Eye T. et al. , (2017). Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood-brain barrier impairment. Proc. Natl. Acad. Sci. U. S. A. 114, E1968–E1976. - PMC - PubMed
-
- Blau C. W., Cowley T. R., O'Sullivan J., Grehan B., Browne T. C., Kelly L., Birch A., Murphy N., Kelly A. M., Kerskens C. M. et al. , (2012). The age-related deficit in LTP is associated with changes in perfusion and blood-brain barrier permeability. Neurobiol. Aging 33, 1005.e23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

