An Inhalable Powder Formulation Based on Micro- and Nanoparticles Containing 5-Fluorouracil for the Treatment of Metastatic Melanoma
- PMID: 29385692
- PMCID: PMC5853707
- DOI: 10.3390/nano8020075
An Inhalable Powder Formulation Based on Micro- and Nanoparticles Containing 5-Fluorouracil for the Treatment of Metastatic Melanoma
Abstract
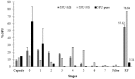
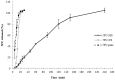
Melanoma is the most aggressive and lethal type of skin cancer, with a poor prognosis because of the potential for metastatic spread. The aim was to develop innovative powder formulations for the treatment of metastatic melanoma based on micro- and nanocarriers containing 5-fluorouracil (5FU) for pulmonary administration, aiming at local and systemic action. Therefore, two innovative inhalable powder formulations were produced by spray-drying using chondroitin sulfate as a structuring polymer: (a) 5FU nanoparticles obtained by piezoelectric atomization (5FU-NS) and (b) 5FU microparticles of the mucoadhesive agent Methocel™ F4M for sustained release produced by conventional spray drying (5FU-MS). The physicochemical and aerodynamic were evaluated in vitro for both systems, proving to be attractive for pulmonary delivery. The theoretical aerodynamic diameters obtained were 0.322 ± 0.07 µm (5FU-NS) and 1.138 ± 0.54 µm (5FU-MS). The fraction of respirable particles (FR%) were 76.84 ± 0.07% (5FU-NS) and 55.01 ± 2.91% (5FU-MS). The in vitro mucoadhesive properties exhibited significant adhesion efficiency in the presence of Methocel™ F4M. 5FU-MS and 5FU-NS were tested for their cytotoxic action on melanoma cancer cells (A2058 and A375) and both showed a cytotoxic effect similar to 5FU pure at concentrations of 4.3 and 1.7-fold lower, respectively.
Keywords: 5-fluorouracil; biopolymers; cytotoxicity; metastatic melanoma; microparticles; nanoparticles; pulmonary delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Melanoma Institute Australia 2017. [(accessed on 13 March 2017)]; Available online: https://www.melanoma.org.au/understanding-melanoma/stages-of-melanoma.
-
- Instituto Nacional de Câncer. [(accessed on 17 July 2017)]; Available online: http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/pele_mel...
-
- Wolff K., Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill; New York, NY, USA: 2009.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

