Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials
- PMID: 29385706
- PMCID: PMC5848909
- DOI: 10.3390/ma11020212
Transparent Glass-Ceramics Produced by Sol-Gel: A Suitable Alternative for Photonic Materials
Abstract
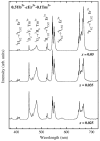
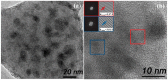
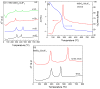
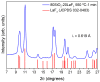
Transparent glass-ceramics have shown interesting optical properties for several photonic applications. In particular, compositions based on oxide glass matrices with fluoride crystals embedded inside, known as oxyfluoride glass-ceramics, have gained increasing interest in the last few decades. Melt-quenching is still the most used method to prepare these materials but sol-gel has been indicated as a suitable alternative. Many papers have been published since the end of the 1990s, when these materials were prepared by sol-gel for the first time, thus a review of the achievements obtained so far is necessary. In the first part of this paper, a review of transparent sol-gel glass-ceramics is made focusing mainly on oxyfluoride compositions. Many interesting optical results have been obtained but very little innovation of synthesis and processing is found with respect to pioneering papers published 20 years ago. In the second part we describe the improvements in synthesis and processing obtained by the authors during the last five years. The main achievements are the preparation of oxyfluoride glass-ceramics with a much higher fluoride crystal fraction, at least double that reported up to now, and the first synthesis of NaGdF₄ glass-ceramics. Moreover, a new SiO₂ precursor was introduced in the synthesis, allowing for a reduction in the treatment temperature and favoring hydroxyl group removal. Interesting optical properties demonstrated the incorporation of dopant ions in the fluoride crystals, thus obtaining crystal-like spectra along with higher efficiencies with respect to xerogels, and hence demonstrating that these materials are a suitable alternative for photonic applications.
Keywords: nanocrystal; optical properties; oxyfluoride glass-ceramics; sol-gel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Feldmann C., Jüstel T., Ronda C.R., Schmidt P.J. Inorganic luminescent materials: 100 years of research and application. Adv. Funct. Mater. 2003;13:511–516. doi: 10.1002/adfm.200301005. - DOI
-
- George N.C., Denault K.A., Seshadri R. Phosphors for solid-state white lightening. Annu. Rev. Mater. Res. 2013;43:481–501. doi: 10.1146/annurev-matsci-073012-125702. - DOI
-
- Huang X. Solid-state lighting: Red phosphor converts white LEDs. Nat. Photonics. 2014;8:748–749. doi: 10.1038/nphoton.2014.221. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

