Colorectal Cancer and Alcohol Consumption-Populations to Molecules
- PMID: 29385712
- PMCID: PMC5836070
- DOI: 10.3390/cancers10020038
Colorectal Cancer and Alcohol Consumption-Populations to Molecules
Erratum in
-
Correction: Rossi et al. Colorectal Cancer and Alcohol Consumption-Populations to Molecules. Cancers 2018, 10, 38.Cancers (Basel). 2024 Nov 29;16(23):3999. doi: 10.3390/cancers16233999. Cancers (Basel). 2024. PMID: 39682311 Free PMC article.
Abstract
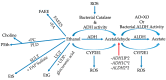
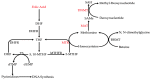
Colorectal cancer (CRC) is a major cause of morbidity and mortality, being the third most common cancer diagnosed in both men and women in the world. Several environmental and habitual factors have been associated with the CRC risk. Alcohol intake, a common and rising habit of modern society, is one of the major risk factors for development of CRC. Here, we will summarize the evidence linking alcohol with colon carcinogenesis and possible underlying mechanisms. Some epidemiologic studies suggest that even moderate drinking increases the CRC risk. Metabolism of alcohol involves ethanol conversion to its metabolites that could exert carcinogenic effects in the colon. Production of ethanol metabolites can be affected by the colon microbiota, another recently recognized mediating factor to colon carcinogenesis. The generation of acetaldehyde and alcohol's other metabolites leads to activation of cancer promoting cascades, such as DNA-adduct formation, oxidative stress and lipid peroxidation, epigenetic alterations, epithelial barrier dysfunction, and immune modulatory effects. Not only does alcohol induce its toxic effect through carcinogenic metabolites, but alcoholics themselves are predisposed to a poor diet, low in folate and fiber, and circadian disruption, which could further augment alcohol-induced colon carcinogenesis.
Keywords: CRC; alcohol; epigenetics; immunity; metabolism; polyposis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Light/Dark Shifting Promotes Alcohol-Induced Colon Carcinogenesis: Possible Role of Intestinal Inflammatory Milieu and Microbiota.Int J Mol Sci. 2016 Dec 2;17(12):2017. doi: 10.3390/ijms17122017. Int J Mol Sci. 2016. PMID: 27918452 Free PMC article.
-
Abnormal Eating Patterns Cause Circadian Disruption and Promote Alcohol-Associated Colon Carcinogenesis.Cell Mol Gastroenterol Hepatol. 2020;9(2):219-237. doi: 10.1016/j.jcmgh.2019.10.011. Epub 2019 Nov 2. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31689559 Free PMC article.
-
Alcohol Effects on Colon Epithelium are Time-Dependent.Alcohol Clin Exp Res. 2019 Sep;43(9):1898-1908. doi: 10.1111/acer.14141. Epub 2019 Jul 22. Alcohol Clin Exp Res. 2019. PMID: 31237690 Free PMC article.
-
Molecular Mechanisms of Alcohol-Induced Colorectal Carcinogenesis.Cancers (Basel). 2021 Aug 31;13(17):4404. doi: 10.3390/cancers13174404. Cancers (Basel). 2021. PMID: 34503214 Free PMC article. Review.
-
Alcohol and Cancer: Epidemiology and Biological Mechanisms.Nutrients. 2021 Sep 11;13(9):3173. doi: 10.3390/nu13093173. Nutrients. 2021. PMID: 34579050 Free PMC article. Review.
Cited by
-
Sex and Tumor-Site Differences in the Association of Alcohol Intake With the Risk of Early-Onset Colorectal Cancer.J Clin Oncol. 2023 Aug 1;41(22):3816-3825. doi: 10.1200/JCO.22.01895. Epub 2023 Jun 14. J Clin Oncol. 2023. PMID: 37315287 Free PMC article.
-
Colorectal cancer screening utilization among breast, cervical, prostate, skin, and lung cancer survivors.J Cancer Surviv. 2024 Apr;18(2):541-552. doi: 10.1007/s11764-022-01258-0. Epub 2022 Oct 10. J Cancer Surviv. 2024. PMID: 36217067 Free PMC article.
-
Curcumin attenuates smoking and drinking activated NF-κB/IL-6 inflammatory signaling axis in cervical cancer.Cancer Cell Int. 2024 Oct 20;24(1):343. doi: 10.1186/s12935-024-03513-z. Cancer Cell Int. 2024. PMID: 39428480 Free PMC article.
-
Global, regional, and national burden of very early-onset colorectal cancer and its risk factors from 1990 to 2019: A systematic analysis for the global burden of disease study 2019.Neoplasia. 2025 Feb;60:101114. doi: 10.1016/j.neo.2024.101114. Epub 2024 Dec 30. Neoplasia. 2025. PMID: 39740538 Free PMC article.
-
Colorectal cancer epidemiology (1990-2050): lessons from the Australasian experience.J Transl Med. 2025 Jul 31;23(1):855. doi: 10.1186/s12967-025-06630-z. J Transl Med. 2025. PMID: 40745565 Free PMC article.
References
-
- Bishehsari F., Mahdavinia M., Vacca M., Malekzadeh R., Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol. 2014;20:6055–6072. doi: 10.3748/wjg.v20.i20.6055. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

