Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine
- PMID: 29385727
- PMCID: PMC5855629
- DOI: 10.3390/ijms19020407
Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine
Abstract
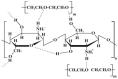
Electrospinning has been used for decades to generate nano-fibres via an electrically charged jet of polymer solution. This process is established on a spinning technique, using electrostatic forces to produce fine fibres from polymer solutions. Amongst, the electrospinning of available biopolymers (silk, cellulose, collagen, gelatine and hyaluronic acid), chitosan (CH) has shown a favourable outcome for tissue regeneration applications. The aim of the current review is to assess the current literature about electrospinning chitosan and its composite formulations for creating fibres in combination with other natural polymers to be employed in tissue engineering. In addition, various polymers blended with chitosan for electrospinning have been discussed in terms of their potential biomedical applications. The review shows that evidence exists in support of the favourable properties and biocompatibility of chitosan electrospun composite biomaterials for a range of applications. However, further research and in vivo studies are required to translate these materials from the laboratory to clinical applications.
Keywords: chitosan; composite solutions; electrospinning; regeneration; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

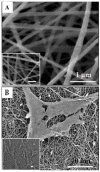

References
-
- Subbiah T., Bhat G.S., Tock R.W., Parameswaran S., Ramkumar S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005;96:557–569. doi: 10.1002/app.21481. - DOI
-
- Sell S.A., Wolfe P.S., Garg K., McCool J.M., Rodriguez I.A., Bowlin G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers. 2010;2:522–553. doi: 10.3390/polym2040522. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

