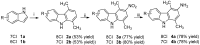Chloro-1,4-dimethyl-9H-carbazole Derivatives Displaying Anti-HIV Activity
- PMID: 29385738
- PMCID: PMC6017966
- DOI: 10.3390/molecules23020286
Chloro-1,4-dimethyl-9H-carbazole Derivatives Displaying Anti-HIV Activity
Abstract
Background: Despite the progress achieved by anti-retroviral drug research in the last decades, the discovery of novel compounds endowed with selective antiviral activity and reduced side effects is still a necessity. At present, the most urgent requirement includes the improvement of HIV (Human Immunodeficiency Virus) prevention and sexual transmission and the development of new drugs to treat the chronic lifelong infection.
Methods: Six chloro-1,4-dimethyl-9H-carbazoles (2a,b-4a,b) have been prepared following opportunely modified known chemical procedures and tested in luciferase and Escherichia coli β-galactosidase expressing CD4⁺, CXCR4⁺, CCR5⁺ TZM-bl cells.
Results and conclusion: a preliminary biological investigation on the synthesized small series of chloro-1,4-dimethyl-9H-carbazoles has been carried out. Among all tested compounds, a nitro-derivative (3b) showed the most interesting profile representing a suitable lead for the development of novel anti-HIV drugs.
Keywords: HIV; antiviral agents; carbazole derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Design, Synthesis and In Vitro Characterization of Novel Antimicrobial Agents Based on 6-Chloro-9H-carbazol Derivatives and 1,3,4-Oxadiazole Scaffolds.Molecules. 2020 Jan 9;25(2):266. doi: 10.3390/molecules25020266. Molecules. 2020. PMID: 31936505 Free PMC article.
-
A novel small molecular weight compound with a carbazole structure that demonstrates potent human immunodeficiency virus type-1 integrase inhibitory activity.Antivir Chem Chemother. 2005;16(6):363-73. doi: 10.1177/095632020501600603. Antivir Chem Chemother. 2005. PMID: 16329284
-
Synthesis of 5,6-dimethyl-9-methoxy-1-phenyl-6H-pyrido[4,3-b]carbazole derivatives and their cytotoxic activity.Arch Pharm (Weinheim). 2005 Nov;338(11):556-61. doi: 10.1002/ardp.200500141. Arch Pharm (Weinheim). 2005. PMID: 16281305
-
HIV chemokine receptor inhibitors as novel anti-HIV drugs.Cytokine Growth Factor Rev. 2005 Dec;16(6):659-77. doi: 10.1016/j.cytogfr.2005.05.009. Epub 2005 Jul 6. Cytokine Growth Factor Rev. 2005. PMID: 16005254 Review.
-
Biological potential of carbazole derivatives.Eur J Med Chem. 2015 Apr 13;94:405-26. doi: 10.1016/j.ejmech.2015.02.059. Epub 2015 Mar 3. Eur J Med Chem. 2015. PMID: 25794500 Review.
Cited by
-
Carbazole Derivatives as Antiviral Agents: An Overview.Molecules. 2019 May 17;24(10):1912. doi: 10.3390/molecules24101912. Molecules. 2019. PMID: 31109016 Free PMC article. Review.
-
Synthesis of novel carbazole hydrazine-carbothioamide scaffold as potent antioxidant, anticancer and antimicrobial agents.BMC Chem. 2024 May 21;18(1):102. doi: 10.1186/s13065-024-01207-1. BMC Chem. 2024. PMID: 38773663 Free PMC article.
-
Discoveries and developments of CXCR4-targeted HIV-1 entry inhibitors.Exp Biol Med (Maywood). 2020 Mar;245(5):477-485. doi: 10.1177/1535370220901498. Epub 2020 Feb 4. Exp Biol Med (Maywood). 2020. PMID: 32019336 Free PMC article. Review.
-
Bis-Thiourea Quaternary Ammonium Salts as Potential Agents against Bacterial Strains from Food and Environmental Matrices.Antibiotics (Basel). 2021 Nov 28;10(12):1466. doi: 10.3390/antibiotics10121466. Antibiotics (Basel). 2021. PMID: 34943678 Free PMC article.
-
Indole - a promising pharmacophore in recent antiviral drug discovery.RSC Med Chem. 2020 Nov 6;11(12):1335-1353. doi: 10.1039/d0md00288g. eCollection 2020 Dec 17. RSC Med Chem. 2020. PMID: 34095843 Free PMC article. Review.
References
-
- NIH Awards Highlight Novel Approaches to HIV Prevention and Treatment. [(accessed on 21 December 2017)]; Available online: https://www.nih.gov/news-events/news-releases/nida-announces-recipients-...
-
- FDA-Approved HIV Medicines. [(accessed on 21 December 2017)]; Available online: https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/58/fda-ap....
-
- Palella F.J., Jr., Baker R.K., Moorman A.C., Chmiel J.S., Wood K.C., Brooks J.T., Holmberg S.D., Investigators H.I.V.O.S. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials