The Effect of Methyl-β-cyclodextrin on Apoptosis, Proliferative Activity, and Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells of Horses Suffering from Metabolic Syndrome (EMS)
- PMID: 29385746
- PMCID: PMC6017619
- DOI: 10.3390/molecules23020287
The Effect of Methyl-β-cyclodextrin on Apoptosis, Proliferative Activity, and Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells of Horses Suffering from Metabolic Syndrome (EMS)
Abstract
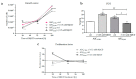
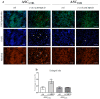
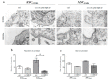
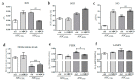
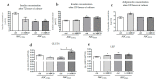
Methyl-β-cyclodextrin (MβCD) is a cyclic oligosaccharide, commonly used as a pharmacological agent to deplete membrane cholesterol. In this study, we examined the effect of MβCD on adipose-derived mesenchymal stromal cells (ASCs) isolated form healthy horses (ASCCTRL) and from horses suffering from metabolic syndrome (ASCEMS). We investigated the changes in the mRNA levels of the glucose transporter 4 (GLUT4) and found that MβCD application may lead to a significant improvement in glucose transport in ASCEMS. We also showed that MβCD treatment affected GLUT4 upregulation in an insulin-independent manner via an NO-dependent signaling pathway. Furthermore, the analysis of superoxide dismutase activity (SOD) and reactive oxygen species (ROS) levels showed that MβCD treatment was associated with an increased antioxidant capacity in ASCEMS. Moreover, we indicated that methyl-β-cyclodextrin treatment did not cause a dysfunction of the endoplasmic reticulum and lysosomes. Thereby, we propose the possibility of improving the functionality of ASCEMS by increasing their metabolic stability.
Keywords: cyclodextrin; equine metabolic syndrome; mesenchymal stem cells; methyl-β-cyclodextrin; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging.Oxid Med Cell Longev. 2016;2016:4710326. doi: 10.1155/2016/4710326. Epub 2015 Nov 22. Oxid Med Cell Longev. 2016. PMID: 26682006 Free PMC article.
-
Orientin Reverses Premature Senescence in Equine Adipose Stromal Cells Affected by Equine Metabolic Syndrome Through Oxidative Stress Modulation.Int J Mol Sci. 2025 Jul 17;26(14):6867. doi: 10.3390/ijms26146867. Int J Mol Sci. 2025. PMID: 40725115 Free PMC article.
-
Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses.Mar Drugs. 2017 Aug 3;15(8):237. doi: 10.3390/md15080237. Mar Drugs. 2017. PMID: 28771165 Free PMC article.
-
Advanced nutritional and stem cells approaches to prevent equine metabolic syndrome.Res Vet Sci. 2018 Jun;118:115-125. doi: 10.1016/j.rvsc.2018.01.015. Epub 2018 Jan 31. Res Vet Sci. 2018. PMID: 29421480 Review.
-
Adipose tissue depots and inflammation: effects on plasticity and resident mesenchymal stem cell function.Cardiovasc Res. 2017 Jul 1;113(9):1064-1073. doi: 10.1093/cvr/cvx096. Cardiovasc Res. 2017. PMID: 28498891 Review.
Cited by
-
Retinoic acid induces the osteogenic differentiation of cat adipose tissue-derived stromal cells from distinct anatomical sites.J Anat. 2023 Feb;242(2):277-288. doi: 10.1111/joa.13758. Epub 2022 Sep 2. J Anat. 2023. PMID: 36056547 Free PMC article.
-
Influence of the Anatomical Site on Adipose Tissue-Derived Stromal Cells' Biological Profile and Osteogenic Potential in Companion Animals.Vet Sci. 2023 Nov 24;10(12):673. doi: 10.3390/vetsci10120673. Vet Sci. 2023. PMID: 38133224 Free PMC article. Review.
-
The Osteogenic Potential of Falciform Ligament-Derived Stromal Cells-A Comparative Analysis between Two Osteogenic Induction Programs.Bioengineering (Basel). 2022 Dec 15;9(12):810. doi: 10.3390/bioengineering9120810. Bioengineering (Basel). 2022. PMID: 36551016 Free PMC article.
References
-
- Marycz K., Kornicka K., Basinska K., Czyrek A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging. Oxid. Med. Cell. Longev. 2016;2016:1–17. doi: 10.1155/2016/4710326. - DOI - PMC - PubMed
-
- Marędziak M., Marycz K., Lewandowski D., Siudzińska A., Śmieszek A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)—A new approach in veterinary regenerative medicine. In Vitro Cell. Dev. Biol. Anim. 2015;51:230–240. doi: 10.1007/s11626-014-9828-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

