Pharmacogenomic Impact of CYP2C19 Variation on Clopidogrel Therapy in Precision Cardiovascular Medicine
- PMID: 29385765
- PMCID: PMC5872082
- DOI: 10.3390/jpm8010008
Pharmacogenomic Impact of CYP2C19 Variation on Clopidogrel Therapy in Precision Cardiovascular Medicine
Abstract
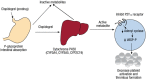
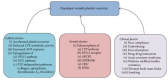
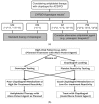
Variability in response to antiplatelet therapy can be explained in part by pharmacogenomics, particularly of the CYP450 enzyme encoded by CYP2C19. Loss-of-function and gain-of-function variants help explain these interindividual differences. Individuals may carry multiple variants, with linkage disequilibrium noted among some alleles. In the current pharmacogenomics era, genomic variation in CYP2C19 has led to the definition of pharmacokinetic phenotypes for response to antiplatelet therapy, in particular, clopidogrel. Individuals may be classified as poor, intermediate, extensive, or ultrarapid metabolizers, based on whether they carry wild type or polymorphic CYP2C19 alleles. Variant alleles differentially impact platelet reactivity, concentration of plasma clopidogrel metabolites, and clinical outcomes. Interestingly, response to clopidogrel appears to be modulated by additional factors, such as sociodemographic characteristics, risk factors for ischemic heart disease, and drug-drug interactions. Furthermore, systems medicine studies suggest that a broader approach may be required to adequately assess, predict, preempt, and manage variation in antiplatelet response. Transcriptomics, epigenomics, exposomics, miRNAomics, proteomics, metabolomics, microbiomics, and mathematical, computational, and molecular modeling should be integrated with pharmacogenomics for enhanced prediction and individualized care. In this review of pharmacogenomic variation of CYP450, a systems medicine approach is described for tailoring antiplatelet therapy in clinical practice of precision cardiovascular medicine.
Keywords: CYP2C19; CYP450; antiplatelet therapy; clopidogrel; genetics variants; pharmacogenomics; precision cardiovascular medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fox C.S., Hall J.L., Arnett D.K., Ashley E.A., Delles C., Engler M.B., Freeman M.W., Johnson J.A., Lanfear D.E., Liggett S.B., et al. Future translational applications from the contemporary genomics era: A scientific statement from the American Heart Association. Circulation. 2015;131:1715–1736. doi: 10.1161/CIR.0000000000000211. - DOI - PMC - PubMed
-
- O’Gara P.T., Kushner F.G., Ascheim D.D., Casey D.E., Chung M.K., de Lemos J.A., Ettinger S.M., Fang J.C., Fesmire F.M., Franklin B.A., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742c84. - DOI - PubMed
-
- Levine G.N., Bates E.R., Blankenship J.C., Bailey S.R., Bittl J.A., Cercek B., Chambers C.E., Ellis S.G., Guyton R.A., Hollenberg S.M., et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574–2609. doi: 10.1161/CIR.0b013e31823a5596. - DOI - PubMed
-
- Rafique A.M., Nayyar P., Wang T.Y., Mehran R., Baber U., Berger P.B., Tobis J., Currier J., Dave R.H., Henry T.D. Optimal P2Y12 Inhibitor in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Network Meta-Analysis. JACC Cardiovasc. Interv. 2016;9:1036–1046. doi: 10.1016/j.jcin.2016.02.013. - DOI - PubMed
-
- Berger J.S., Bhatt D.L., Cannon C.P., Chen Z., Jiang L., Jones J.B., Mehta S.R., Sabatine M.S., Steinhubl S.R., Topol E.J., et al. The relative efficacy and safety of clopidogrel in women and men a sex-specific collaborative meta-analysis. J. Am. Coll. Cardiol. 2009;54:1935–1945. doi: 10.1016/j.jacc.2009.05.074. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

