Moonlighting with WDR5: A Cellular Multitasker
- PMID: 29385767
- PMCID: PMC5852437
- DOI: 10.3390/jcm7020021
Moonlighting with WDR5: A Cellular Multitasker
Abstract
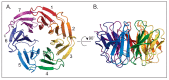
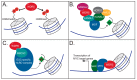
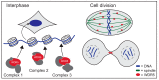
WDR5 is a highly conserved WD40 repeat-containing protein that is essential for proper regulation of multiple cellular processes. WDR5 is best characterized as a core scaffolding component of histone methyltransferase complexes, but emerging evidence demonstrates that it does much more, ranging from expanded functions in the nucleus through to controlling the integrity of cell division. The purpose of this review is to describe the current molecular understandings of WDR5, discuss how it participates in diverse cellular processes, and highlight drug discovery efforts around WDR5 that may form the basis of new anti-cancer therapies.
Keywords: WD40 repeat; WDR5; cancer; drug discovery; epigenetics; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Recent Progress in Modulation of WD40-Repeat Domain 5 Protein (WDR5): Inhibitors and Degraders.Cancers (Basel). 2023 Aug 1;15(15):3910. doi: 10.3390/cancers15153910. Cancers (Basel). 2023. PMID: 37568727 Free PMC article. Review.
-
Molecular recognition of histone H3 by the WD40 protein WDR5.Nat Struct Mol Biol. 2006 Aug;13(8):698-703. doi: 10.1038/nsmb1116. Epub 2006 Jul 9. Nat Struct Mol Biol. 2006. PMID: 16829960
-
WDR5 positively regulates p53 stability by inhibiting p53 ubiquitination.Biochem Biophys Res Commun. 2017 May 27;487(2):333-338. doi: 10.1016/j.bbrc.2017.04.060. Epub 2017 Apr 13. Biochem Biophys Res Commun. 2017. PMID: 28412363
-
WDR5 in porcine preimplantation embryos: expression, regulation of epigenetic modifications and requirement for early development†.Biol Reprod. 2017 Apr 1;96(4):758-771. doi: 10.1093/biolre/iox020. Biol Reprod. 2017. PMID: 28379447
-
Diverse roles of WDR5-RbBP5-ASH2L-DPY30 (WRAD) complex in the functions of the SET1 histone methyltransferase family.J Biosci. 2017 Mar;42(1):155-159. doi: 10.1007/s12038-017-9666-9. J Biosci. 2017. PMID: 28229975 Review.
Cited by
-
Interaction of the oncoprotein transcription factor MYC with its chromatin cofactor WDR5 is essential for tumor maintenance.Proc Natl Acad Sci U S A. 2019 Dec 10;116(50):25260-25268. doi: 10.1073/pnas.1910391116. Epub 2019 Nov 25. Proc Natl Acad Sci U S A. 2019. PMID: 31767764 Free PMC article.
-
WDR5 represents a therapeutically exploitable target for cancer stem cells in glioblastoma.Genes Dev. 2023 Feb 1;37(3-4):86-102. doi: 10.1101/gad.349803.122. Epub 2023 Feb 2. Genes Dev. 2023. PMID: 36732025 Free PMC article.
-
A novel TOX3-WDR5-ABCG2 signaling axis regulates the progression of colorectal cancer by accelerating stem-like traits and chemoresistance.PLoS Biol. 2023 Sep 14;21(9):e3002256. doi: 10.1371/journal.pbio.3002256. eCollection 2023 Sep. PLoS Biol. 2023. PMID: 37708089 Free PMC article.
-
Kinetics of the multitasking high-affinity Win binding site of WDR5 in restricted and unrestricted conditions.Biochem J. 2021 Jun 11;478(11):2145-2161. doi: 10.1042/BCJ20210253. Biochem J. 2021. PMID: 34032265 Free PMC article.
-
Unannotated microprotein EMBOW regulates the interactome and chromatin and mitotic functions of WDR5.Cell Rep. 2023 Sep 26;42(9):113145. doi: 10.1016/j.celrep.2023.113145. Epub 2023 Sep 19. Cell Rep. 2023. PMID: 37725512 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

