Pretreatment quality of life as a predictor of survival for patients with nasopharyngeal carcinoma treated with IMRT
- PMID: 29386004
- PMCID: PMC5793429
- DOI: 10.1186/s12885-018-4003-8
Pretreatment quality of life as a predictor of survival for patients with nasopharyngeal carcinoma treated with IMRT
Abstract
Background: To evaluate the prognostic significance of pretreatment quality of life for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy.
Methods: We performed a prospective, longitudinal study on 554 newly diagnosed patients with NPC from April 2011 to January 2015. A total of 501 consecutive NPC patients were included. Patients were asked to complete the EORTC QLQ-C30 (version 3.0) and QLQ-H&N35 questionnaires before treatment.
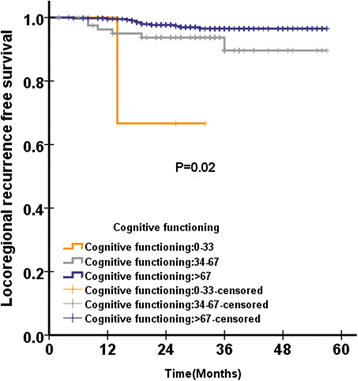
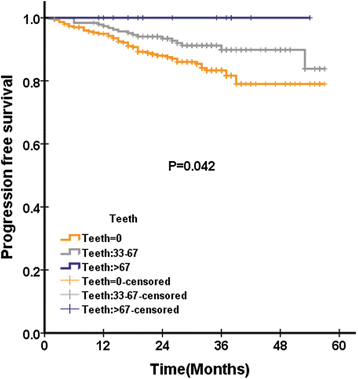
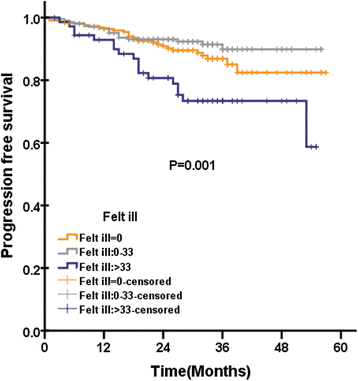
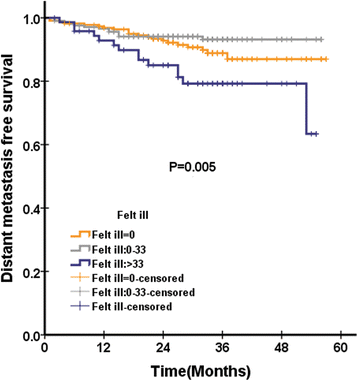
Results: Global health status among QLQ-C30 correlates with EBV DNA(P = 0.019). In addition, pretreatment appetite loss was significantly correlated with EBV DNA(P = 0.02). Pretreatment teeth, opening mouth, feeding tube was significantly correlated with EBV DNA, with P value of 0.003, < 0.0001, and 0.031, respectively. In multivariate analysis, pretreatment cognitive functioning of QLQ-C30 was significantly associated with LRFS, with HR of 0.971(95%CI 0.951-0.990), P = 0.004. Among scales of QLQ-H&N35 for multivariate analysis, pretreatment teeth (P = 0.026) and felt ill (P = 0.012) was significantly associated with PFS, with HR of 0.984 (95%CI 0.971-.998) and 1.004 (95%CI 1.001-1.007), respectively. Felt ill of QLQ-H&N35 was significantly associated with DMFS, with HR of 1.004(95%CI 1.000-1.007), P = 0.043. There is no QoL scale significantly associated with OS after multivariate analysis.
Conclusions: In conclusion, our analysis confirms that pretreatment teeth and felt ill was significantly associated with PFS in NPC patients treated with IMRT. In addition, the posttreatment EBV DNA was significantly associated with OS.
Keywords: EBV DNA; Nasopharyngeal carcinoma; Prognostic factor; Quality of life; Survival.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the clinical research ethics committee of the Sun Yat-Sen University Cancer Center(B2011–004-01), and the participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Wang R, Wu F, Lu H, Wei B, Feng G, Li G, Liu M, Yan H, Zhu J, Zhang Y, et al. Definitive intensity-modulated radiation therapy for nasopharyngeal carcinoma: long-term outcome of a multicenter prospective study. J Cancer Res Clin Oncol. 2013;139(1):139–145. doi: 10.1007/s00432-012-1313-0. - DOI - PMC - PubMed
-
- Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13(2):163–171. doi: 10.1016/S1470-2045(11)70320-5. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- 81425018/National Natural Science Foundation of China/International
- 81072226/National Natural Science Foundation of China/International
- 81201629/National Natural Science Foundation of China/International
- 2012AA02A501/the 863 Project/International
- 2013CB910304/National Key Basic Research Program of China/International
- 2014TX01R145/Special Support Plan of Guangdong Province/International
- No.2014A020212103, No.2011B080701034, No.2011B031800161/Sci-Tech Project Foundation of Guangdong Province/International
- 201400000001/Health & Medical Collaborative Innovation Project of Guangzhou City/International
- 2014BAI09B10/National Science & Technology Pillar Program during the Twelfth Five-year Plan Period/International
- A2014252/the Medical Research Foundation of Guangdong Province/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

