Long non-coding RNA XLOC_000647 suppresses progression of pancreatic cancer and decreases epithelial-mesenchymal transition-induced cell invasion by down-regulating NLRP3
- PMID: 29386037
- PMCID: PMC5793431
- DOI: 10.1186/s12943-018-0761-9
Long non-coding RNA XLOC_000647 suppresses progression of pancreatic cancer and decreases epithelial-mesenchymal transition-induced cell invasion by down-regulating NLRP3
Abstract
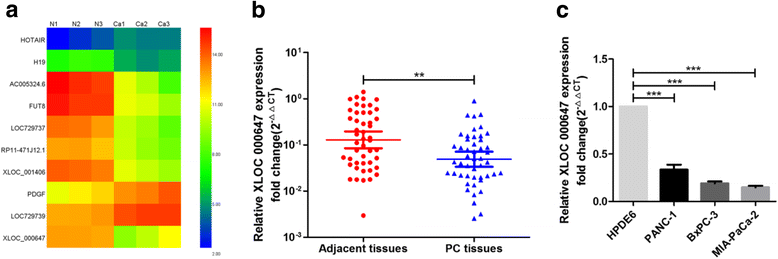
Background: Long non-coding RNAs (lncRNAs) play an important role in the development and progression of various tumors, including pancreatic cancer (PC). Recent studies have shown that lncRNAs can 'act in cis' to regulate the expression of its neighboring genes. Previously, we used lncRNAs microarray to identify a novel lncRNA termed XLOC_000647 that was down-regulated in PC tissues. However, the expression and function of XLOC_000647 in PC remain unclear.
Methods: The expression of XLOC_000647 and NLRP3 in PC specimens and cell lines were detected by quantitative real-time PCR. Transwell assays were used to determine migration and invasion of PC cells. Western blot was carried out for detection of epithelial-mesenchymal transition (EMT) markers in PC cells. The effect of XLOC_000647 on PC cells was assessed in vitro and in vivo. The function of NOD-like receptor family pyrin domain-containing 3 (NLRP3) in PC was investigated in vitro. In addition, the regulation of NLRP3 by XLOC_000647 in PC was examined in vitro.
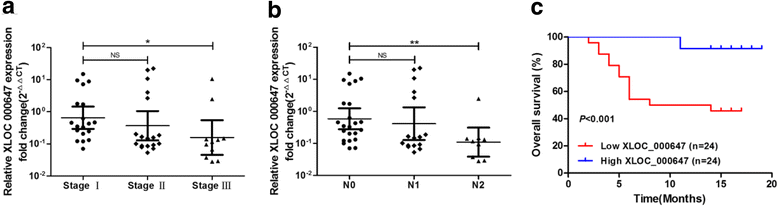
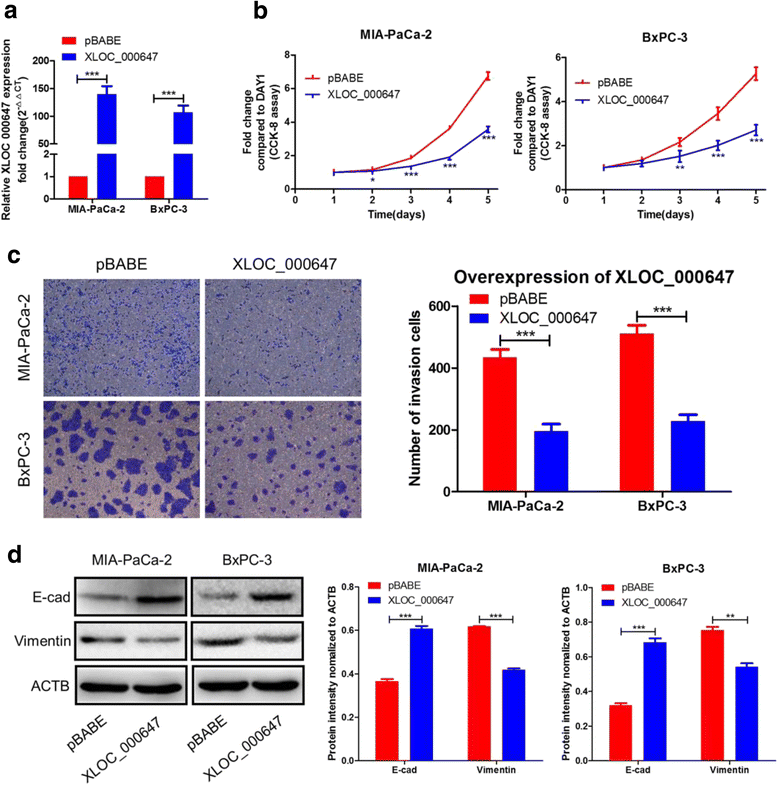
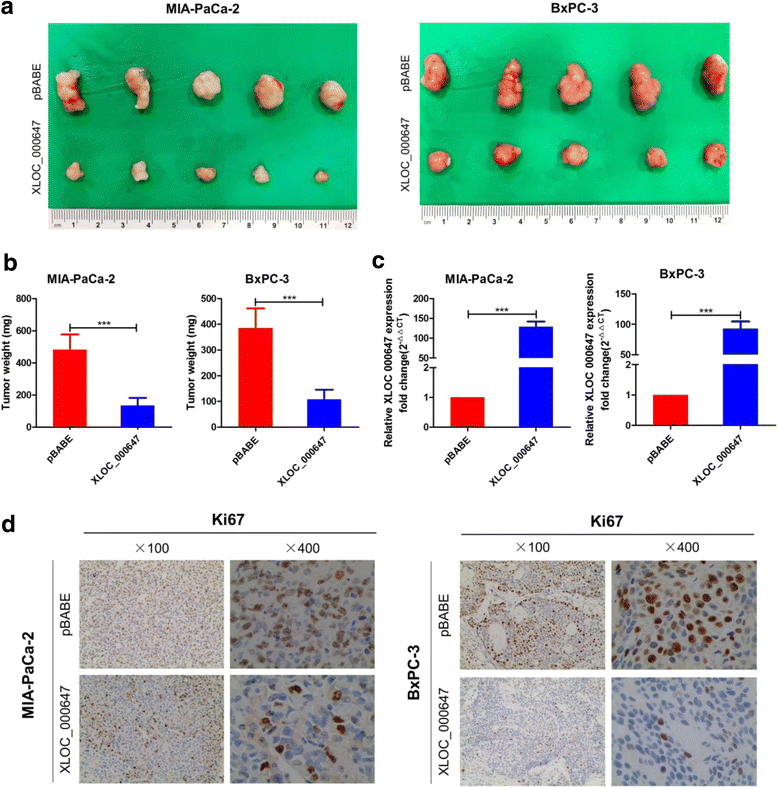
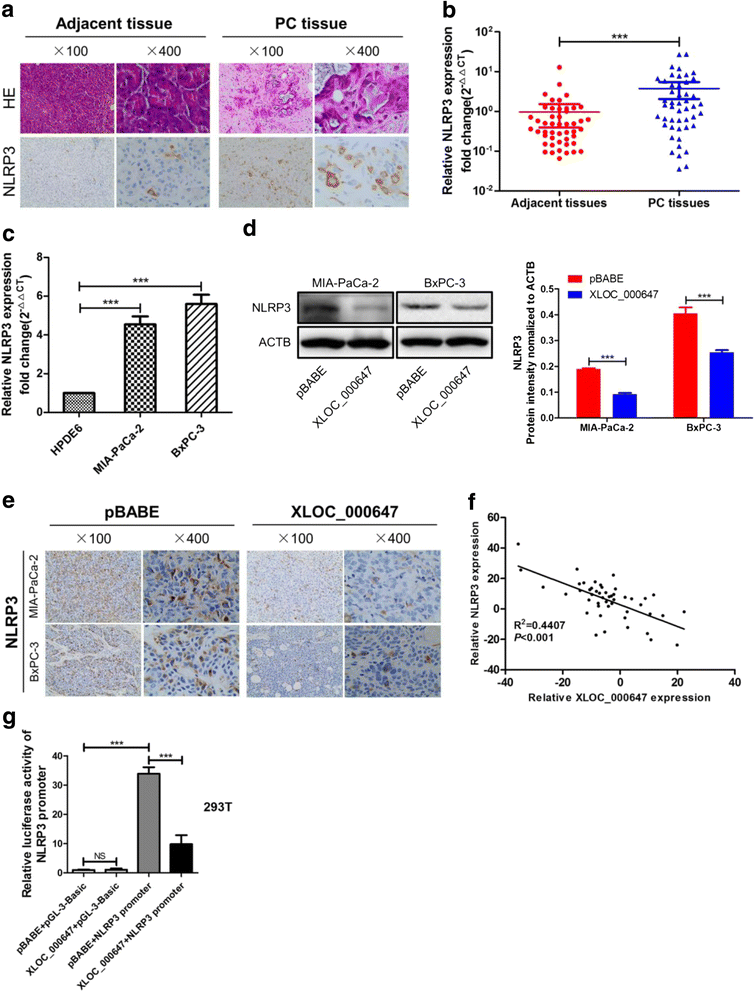
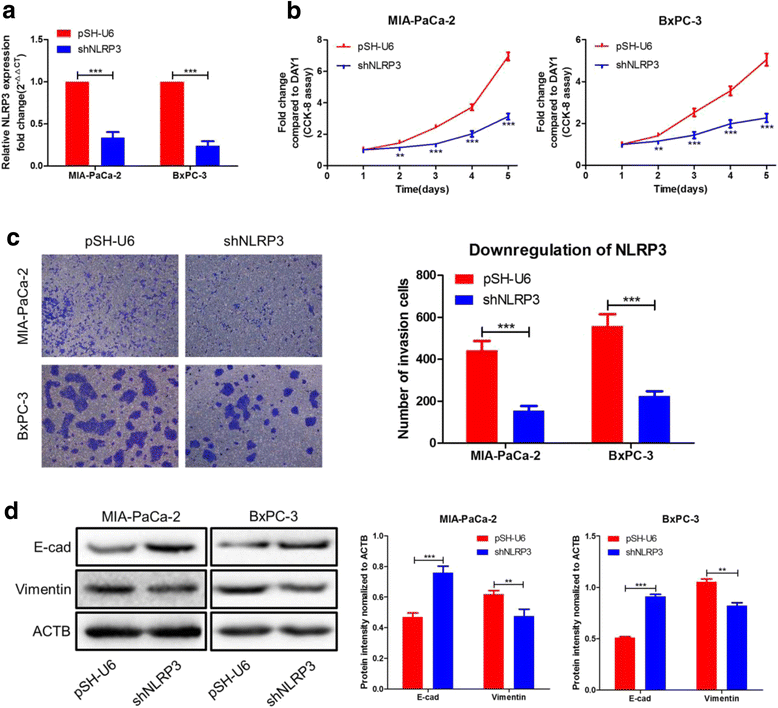
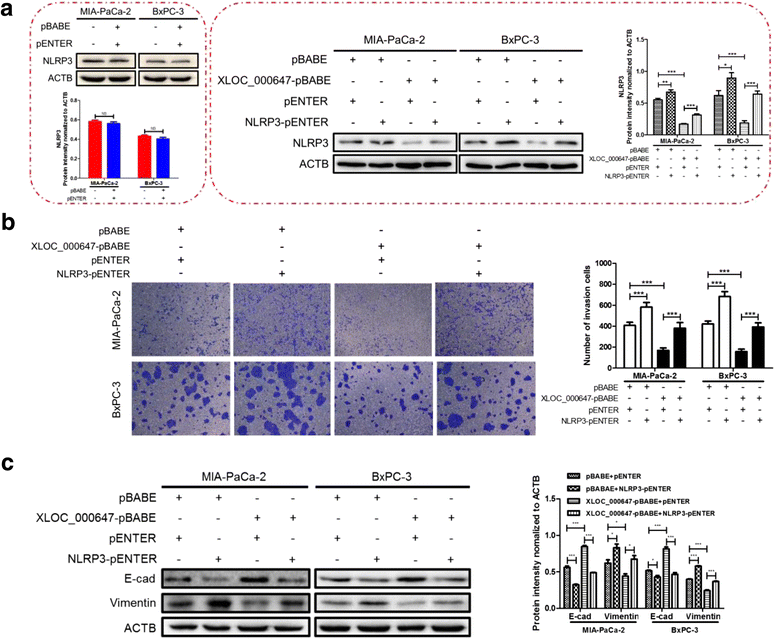
Results: Here, XLOC_000647 expression was down-regulated in PC tissues and cell lines. The expression level of XLOC_000647 was significantly correlated to tumor stage, lymph node metastasis, and overall survival. Overexpression of XLOC_000647 attenuated cell proliferation, invasion, and EMT in vitro and impaired tumor growth in vivo. Further, a significantly negative correlation was observed between XLOC_000647 levels and its genomic nearby gene NLRP3 in vitro and in vivo. Moreover, XLOC_000647 decreased NLRP3 by inhibiting its promoter activity. Knockdown of NLRP3 decreased proliferation of cancer cells, invasion, and EMT in vitro. Importantly, after XLOC_000647 was overexpressed, the corresponding phenotypes of cells invasion and EMT were reversed by overexpression of NLRP3.
Conclusions: Together, these results indicate that XLOC_000647 functions as a novel tumor suppressor of lncRNA and acts as an important regulator of NLRP3, inhibiting cell proliferation, invasion, and EMT in PC.
Keywords: Epithelial mesenchymal transition; Invasion; LncRNAs; NLRP3; Pancreatic cancer.
Conflict of interest statement
Ethics approval and consent to participate
The clinical protocol was approved by the Ethics Committee of The First Hospital Affiliated to Nanjing Medical University and all patients signed a written informed consent form before specimen collection. The animal care and experimental protocols were approved by the institutional guidelines of Jiangsu Province and by the Animal Care and Use Committee of Nanjing Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Up-regulation of long non-coding RNA XLOC_010235 regulates epithelial-to-mesenchymal transition to promote metastasis by associating with Snail1 in gastric cancer.Sci Rep. 2017 May 26;7(1):2461. doi: 10.1038/s41598-017-02254-6. Sci Rep. 2017. PMID: 28550287 Free PMC article.
-
Long non-coding RNA GHET1 promotes human breast cancer cell proliferation, invasion and migration via affecting epithelial mesenchymal transition.Cancer Biomark. 2018;22(3):565-573. doi: 10.3233/CBM-181250. Cancer Biomark. 2018. PMID: 29843220
-
Knockdown of lncRNA GHET1 inhibits osteosarcoma cells proliferation, invasion, migration and EMT in vitro and in vivo.Cancer Biomark. 2018;23(4):589-601. doi: 10.3233/CBM-181863. Cancer Biomark. 2018. PMID: 30475755
-
Regulation of NLRP3 by non-coding RNAs in different cancers: interplay between non-coding RNAs and NLRP3 in carcinogenesis and metastasis.Cell Mol Biol (Noisy-le-grand). 2020 Dec 31;66(8):47-51. Cell Mol Biol (Noisy-le-grand). 2020. PMID: 34174977 Review.
-
Long Noncoding RNA and Epithelial Mesenchymal Transition in Cancer.Int J Mol Sci. 2019 Apr 18;20(8):1924. doi: 10.3390/ijms20081924. Int J Mol Sci. 2019. PMID: 31003545 Free PMC article. Review.
Cited by
-
PCDH1, a poor prognostic biomarker and potential target for pancreatic adenocarcinoma metastatic therapy.BMC Cancer. 2023 Nov 13;23(1):1102. doi: 10.1186/s12885-023-11474-1. BMC Cancer. 2023. PMID: 37957639 Free PMC article.
-
Pancreatic ductal adenocarcinoma immune microenvironment and immunotherapy prospects.Chronic Dis Transl Med. 2020 Feb 11;6(1):6-17. doi: 10.1016/j.cdtm.2020.01.002. eCollection 2020 Mar. Chronic Dis Transl Med. 2020. PMID: 32226930 Free PMC article. Review.
-
LncRNA ZNFTR functions as an inhibitor in pancreatic cancer by modulating ATF3/ZNF24/VEGFA pathway.Cell Death Dis. 2021 Sep 3;12(9):830. doi: 10.1038/s41419-021-04119-3. Cell Death Dis. 2021. PMID: 34480024 Free PMC article.
-
Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: Role of lncRNAs in cytokine storm modulation.Life Sci. 2020 Sep 15;257:118114. doi: 10.1016/j.lfs.2020.118114. Epub 2020 Jul 18. Life Sci. 2020. PMID: 32693241 Free PMC article. Review.
-
Overcoming Immunotherapy Resistance by Targeting the Tumor-Intrinsic NLRP3-HSP70 Signaling Axis.Cancers (Basel). 2021 Sep 23;13(19):4753. doi: 10.3390/cancers13194753. Cancers (Basel). 2021. PMID: 34638239 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 81272382/National Natural Science Foundation of China/International
- 81672449/National Natural Science Foundation of China/International
- BZ2016788/Social Development of Science and Technology Research Projects of Jiangsu Province/International
- 2012-16/Blue Project of Colleges and Universities of Jiangsu Province/International
- JX10231801/Priority Academic Program Development of Jiangsu Higher Education Institutions/International
- BM2015004/Innovation Capability Development Project of Jiangsu Province/International
- ZDXKA2016005/Jiangsu Key Medical Discipline (General Surgery)/International
- No. QNRC015/Special Funding of Science and Education Enhancing Health of Wuxi/International
- Q201621/Young Researcher Grants Program of Wuxi Health and Family Planning Commission/International
- CSZ0N1709/Science and Technology Bureau guidance project of Wuxi/International
- 2016-2018-10221/Medical Core Member Science Fund of The Third Hospital Affiliated to Nantong University/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

