Characteristics of drugs for ultra-rare diseases versus drugs for other rare diseases in HTA submissions made to the CADTH CDR
- PMID: 29386040
- PMCID: PMC5793441
- DOI: 10.1186/s13023-018-0762-1
Characteristics of drugs for ultra-rare diseases versus drugs for other rare diseases in HTA submissions made to the CADTH CDR
Abstract
Background: It has been suggested that ultra-rare diseases should be recognized as distinct from more prevalent rare diseases, but how drugs developed to treat ultra-rare diseases (DURDs) might be distinguished from drugs for 'other' rare diseases (DORDs) is not clear. We compared the characteristics of DURDs to DORDs from a health technology assessment (HTA) perspective in submissions made to the CADTH Common Drug Review. We defined a DURD as a drug used to treat a disease with a prevalence ≤ 1 patient per 100,000 people, a DORD as a drug used to treat a disease with a prevalence > 1 and ≤ 50 patients per 100,000 people. We assessed differences in the level and quantity of evidence supporting each HTA submission, the molecular basis of treatment agents, annual treatment cost per patient, type of reimbursement recommendation made by CADTH, and reasons for negative recommendations.
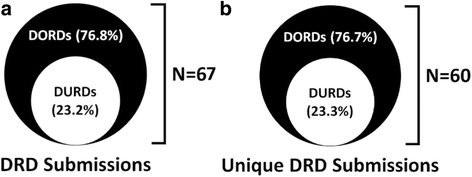
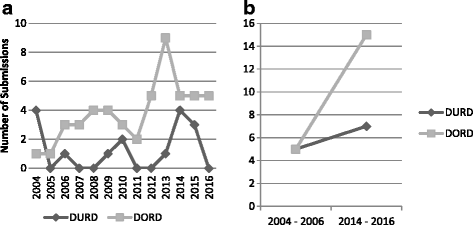
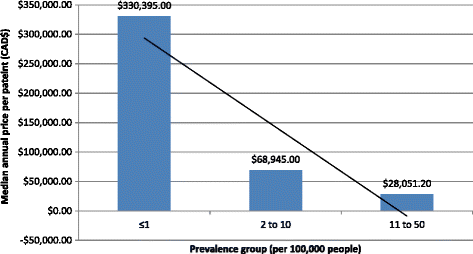
Results: We analyzed 14 DURD and 46 DORD submissions made between 2004 and 2016. Compared to DORDs, DURDs were more likely to be biologic drugs (OR = 6.06, 95%CI 1.25 to 38.58), to have been studied in uncontrolled clinical trials (OR = 23.11, 95%CI 2.23 to 1207.19), and to have a higher annual treatment cost per patient (median difference = CAN$243,787.75, 95%CI CAN$83,396 to CAN$329,050). Also, submissions for DURDs were associated with a less robust evidence base versus DORDs, as DURD submissions were less likely to include data from at least one double-blinded randomized controlled trial (OR = 0.13, 95%CI 0.02 to 0.70) and have smaller patient cohorts in clinical trials (median difference = -108, 95%CI -234 to -50). Furthermore, DURDs are less likely to receive a positive reimbursement recommendation (OR = 0.22, 95%CI 0.05 to 0.91), and low level of evidence was the major contributor for a negative recommendation.
Conclusions: The results suggest that DURDs could be viewed as distinct category from an HTA perspective. Applying the same HTA decision-making framework to DURDs and DORDs might have contributed the higher rate of negative reimbursement recommendations made for DURDs. Recognition of DURDs as a distinct subgroup of DRDs by explicitly defining DURDs based on objective criteria may facilitate the implementation of HTA assessment process that accounts for the issues associated with DURD.
Keywords: Canada; Orphan drugs; Rare diseases; Technology assessment, health; Ultra-rare diseases.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hadjivasiliou A. Orphan drug report 2015. EvaluatePharma; 2015 October 2015.
-
- Tordrup D, Tzouma V, Kanavos P. Orphan drug considerations in health technology assessment in eight European countries. Rare Dis Orphan Drugs: Intl J Public Health. 2014;1(3):86–97.
-
- Richter T, Nestler-Parr S, Babela R, Khan ZM, Tesoro T, Molsen E, et al. Rare disease terminology and definitions-a systematic global review: report of the ISPOR rare disease special interest group. Value in Health: J Int Soc Pharmacoeco Outcomes Res 2015;18(6):906-914. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

