Histopathological and immunohistochemical characterisation of hepatic granulomas in Leishmania donovani-infected BALB/c mice: a time-course study
- PMID: 29386047
- PMCID: PMC5793367
- DOI: 10.1186/s13071-018-2624-z
Histopathological and immunohistochemical characterisation of hepatic granulomas in Leishmania donovani-infected BALB/c mice: a time-course study
Abstract
Background: Visceral leishmaniasis (VL) is a neglected tropical disease (NTD), caused by the intracellular protozoan parasites Leishmania donovani and Leishmania infantum. Symptomatic VL is considered fatal when left untreated. At present, there is no effective vaccine licensed for human use and available chemotherapies have limitations. Understanding the local immune mechanisms required for the control of infection is a key factor for developing effective vaccines and therapeutics.
Methods: We have investigated the development of the typical granulomatous lesions in the liver in experimental VL over time, together with the local immune responses. BALB/c mice were infected intravenously with a dose of 2 × 107 L. donovani amastigotes (MHOM/ET/67/HU3) and sacrificed at 15, 35 and 63 days post-infection (dpi). Histopathology and immunohistochemical techniques were used for the detection of Leishmania antigen, selected cell types including B and T lymphocytes, macrophages and neutrophils (CD45R-B220+, CD3+, F4/80+ and Ly-6G+) and iNOS.
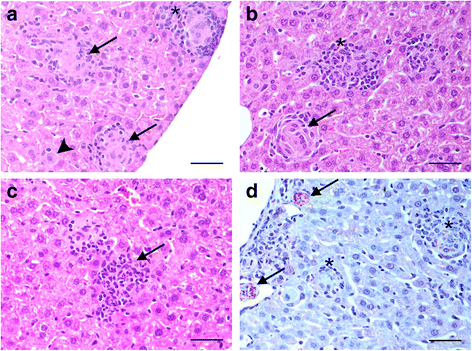
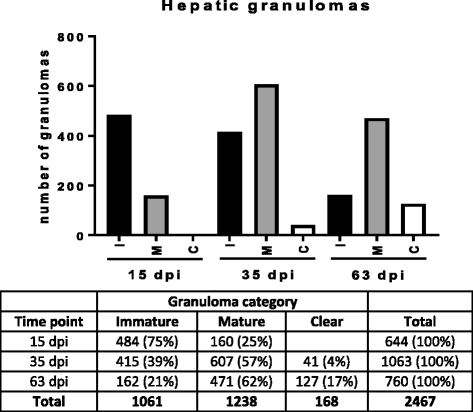
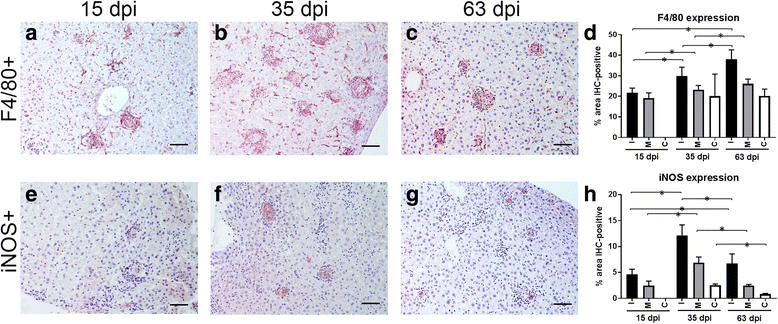
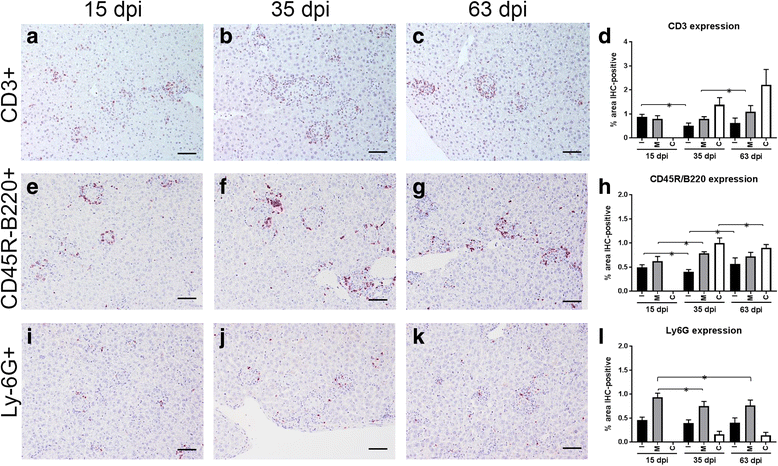
Results: Granulomatous lesions were identified as early as 15 dpi in the livers of all infected animals. Three categories were used to classify liver granulomas (immature, mature and clear). Clear granulomas were exclusively detected from 35 dpi onwards. Kupffer cells (F4/80+) were predominant in immature granulomas, regardless of the dpi. Nonetheless, the highest expression was found 63 dpi. Positive staining for iNOS was mainly observed in the cytoplasm of fused Kupffer cells and the highest expression observed at 35 dpi. T cells (CD3+) and B cells (CD45R-B220+) were predominant in more advanced granuloma stages, probably related to the establishment of acquired immunity. Neutrophils (Ly-6G+) were predominantly observed in mature granulomas with the highest expression at 15 dpi. Neutrophils were lower in numbers compared to other cell types, particularly at later time points.
Conclusions: Our results reflect the role of macrophages during the early stage of infection and the establishment of a lymphocytic response to control the infection in more advanced stages.
Keywords: Granuloma; Histopathology; Immunohistochemistry; Leishmania donovani; Macrophage; Visceral leishmaniasis.
Conflict of interest statement
Ethics approval
All animal experiments were approved by the Animal Welfare and Ethics Review Board at LSHTM and performed under license in accordance with the Animals (Scientific Procedures) Act of 1986 (UK Home Office project license PPL70/8207).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

