Curcumin-mediated bone marrow mesenchymal stem cell sheets create a favorable immune microenvironment for adult full-thickness cutaneous wound healing
- PMID: 29386050
- PMCID: PMC5793416
- DOI: 10.1186/s13287-018-0768-6
Curcumin-mediated bone marrow mesenchymal stem cell sheets create a favorable immune microenvironment for adult full-thickness cutaneous wound healing
Abstract
Background: Adult full-thickness cutaneous wound repair suffers from an imbalanced immune response, leading to nonfunctional reconstructed tissue and fibrosis. Although various treatments have been reported, the immune-mediated tissue regeneration driven by biomaterial offers an attractive regenerative strategy for damaged tissue repair.
Methods: In this research, we investigated a specific bone marrow-derived mesenchymal stem cell (BMSC) sheet that was induced by the Traditional Chinese Medicine curcumin (CS-C) and its immunomodulatory effects on wound repair. Comparisons were made with the BMSC sheet induced without curcumin (CS-N) and control (saline).
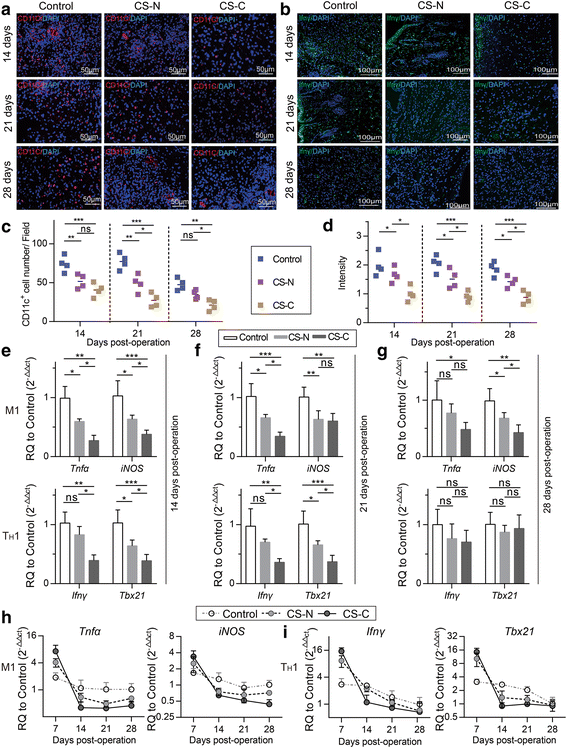
Results: In vitro cultured BMSC sheets (CS-C) showed that curcumin promoted the proliferation of BMSCs and modified the features of produced extracellular matrix (ECM) secreted by BMSCs, especially the contents of ECM structural proteins such as fibronectin (FN) and collagen I and III, as well as the ratio of collagen III/I. Two-photon fluorescence (TPF) and second-harmonic generation (SHG) imaging of mouse implantation revealed superior engraftment of BMSCs, maintained for 35 days in the CS-C group. Most importantly, CS-C created a favorable immune microenvironment. The chemokine stromal cell-derived factor 1 (SDF1) was abundantly produced by CS-C, thus facilitating a mass migration of leukocytes from which significantly increased expression of signature TH1 cells (interferon gamma) and M1 macrophages (tumor necrosis factor alpha) genes were confirmed at 7 days post-operation. The number of TH1 cells and associated pro-inflammatory M1 macrophages subsequently decreased sharply after 14 days post-operation, suggesting a rapid type I immune regression. Furthermore, the CS-C group showed an increased trend towards M2 macrophage polarization in the early phase. CS-C led to an epidermal thickness and collagen deposition that was closer to that of normal skin.
Conclusions: Curcumin has a good regulatory effect on BMSCs and this promising CS-C biomaterial creates a pro-regenerative immune microenvironment for cutaneous wound healing.
Keywords: Bone marrow mesenchymal stem cell; Cell sheet; Curcumin; Cutaneous wound healing; Immune response.
Conflict of interest statement
Ethics approval
All experimental protocols were approved by the Ethical Committee for Animal Experiments of South China Normal University. All animal experiments conducted in this research were performed in accordance with the guidelines of South China Normal University Intramural Animal Use and Care Committee and met the NIH guidelines for the care and use of laboratory animals.
Consent for publication
All authors have read and approved the manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures








Similar articles
-
Hypoxic preconditioning combined with curcumin promotes cell survival and mitochondrial quality of bone marrow mesenchymal stem cells, and accelerates cutaneous wound healing via PGC-1α/SIRT3/HIF-1α signaling.Free Radic Biol Med. 2020 Nov 1;159:164-176. doi: 10.1016/j.freeradbiomed.2020.07.023. Epub 2020 Jul 31. Free Radic Biol Med. 2020. PMID: 32745765
-
Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization.Med Sci Monit. 2020 May 5;26:e923328. doi: 10.12659/MSM.923328. Med Sci Monit. 2020. PMID: 32369458 Free PMC article.
-
Combining mesenchymal stem cell sheets with platelet-rich plasma gel/calcium phosphate particles: a novel strategy to promote bone regeneration.Stem Cell Res Ther. 2015 Dec 21;6:256. doi: 10.1186/s13287-015-0256-1. Stem Cell Res Ther. 2015. PMID: 26689714 Free PMC article.
-
Bone marrow-derived stem cells in wound healing: a review.Wound Repair Regen. 2007 Sep-Oct;15 Suppl 1:S18-26. doi: 10.1111/j.1524-475X.2007.00221.x. Wound Repair Regen. 2007. PMID: 17727462 Review.
-
Healing the Broken Heart; The Immunomodulatory Effects of Stem Cell Therapy.Front Immunol. 2020 Apr 9;11:639. doi: 10.3389/fimmu.2020.00639. eCollection 2020. Front Immunol. 2020. PMID: 32328072 Free PMC article. Review.
Cited by
-
Mesenchymal stem cell-based bioengineered constructs: foreign body response, cross-talk with macrophages and impact of biomaterial design strategies for pelvic floor disorders.Interface Focus. 2019 Aug 6;9(4):20180089. doi: 10.1098/rsfs.2018.0089. Epub 2019 Jun 14. Interface Focus. 2019. PMID: 31263531 Free PMC article. Review.
-
Epithelial differentiation of gingival mesenchymal stem cells enhances re-epithelialization for full-thickness cutaneous wound healing.Stem Cell Res Ther. 2024 Nov 28;15(1):455. doi: 10.1186/s13287-024-04081-9. Stem Cell Res Ther. 2024. PMID: 39609719 Free PMC article.
-
Endothelial progenitor cells improve the therapeutic effect of mesenchymal stem cell sheets on irradiated bone defect repair in a rat model.J Transl Med. 2018 May 22;16(1):137. doi: 10.1186/s12967-018-1517-4. J Transl Med. 2018. PMID: 29788957 Free PMC article.
-
A Comprehensive Review: Advances in Mesenchymal Stem Cell Applications for Burn Wound Repair.Stem Cells Int. 2025 Mar 20;2025:6683745. doi: 10.1155/sci/6683745. eCollection 2025. Stem Cells Int. 2025. PMID: 40151391 Free PMC article. Review.
-
Human Salivary Histatin-1 Is More Efficacious in Promoting Acute Skin Wound Healing Than Acellular Dermal Matrix Paste.Front Bioeng Biotechnol. 2020 Aug 19;8:999. doi: 10.3389/fbioe.2020.00999. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32974320 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

