Myoblast fusion confusion: the resolution begins
- PMID: 29386054
- PMCID: PMC5793351
- DOI: 10.1186/s13395-017-0149-3
Myoblast fusion confusion: the resolution begins
Abstract
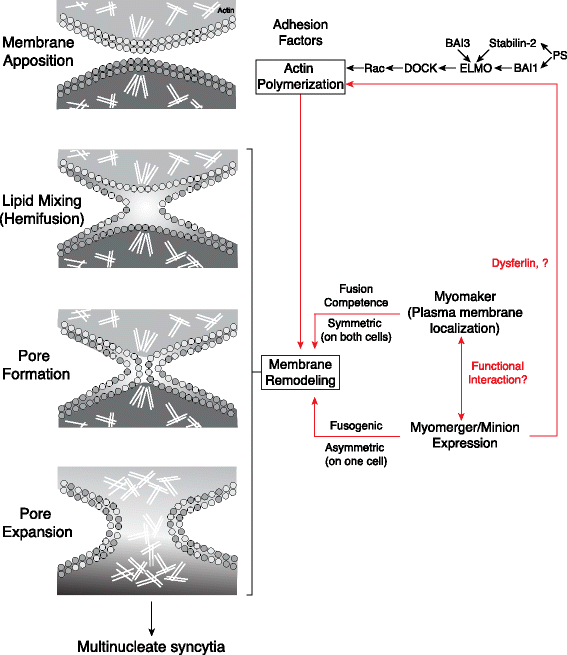
The fusion of muscle precursor cells is a required event for proper skeletal muscle development and regeneration. Numerous proteins have been implicated to function in myoblast fusion; however, the majority are expressed in diverse tissues and regulate numerous cellular processes. How myoblast fusion is triggered and coordinated in a muscle-specific manner has remained a mystery for decades. Through the discovery of two muscle-specific fusion proteins, Myomaker and Myomerger-Minion, we are now primed to make significant advances in our knowledge of myoblast fusion. This article reviews the latest findings regarding the biology of Myomaker and Minion-Myomerger, places these findings in the context of known pathways in mammalian myoblast fusion, and highlights areas that require further investigation. As our understanding of myoblast fusion matures so does our potential ability to manipulate cell fusion for therapeutic purposes.
Keywords: Minion; Muscle development; Myoblast fusion; Myomaker; Myomerger.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Sh.C.S. and Sn.C.S. are employees of the Novartis Institutes for BioMedical Research.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

