Delivery of VEGFA in bone marrow stromal cells seeded in copolymer scaffold enhances angiogenesis, but is inadequate for osteogenesis as compared with the dual delivery of VEGFA and BMP2 in a subcutaneous mouse model
- PMID: 29386057
- PMCID: PMC5793460
- DOI: 10.1186/s13287-018-0778-4
Delivery of VEGFA in bone marrow stromal cells seeded in copolymer scaffold enhances angiogenesis, but is inadequate for osteogenesis as compared with the dual delivery of VEGFA and BMP2 in a subcutaneous mouse model
Abstract
Background: In bone tissue engineering (BTE), extensive research into vascular endothelial growth factor A (VEGFA)-mediated angiogenesis has yielded inconsistent results. The aim of this study was to investigate the influence on angio- and osteogenesis of adenoviral-mediated delivery of VEGFA alone or in combination with bone morphogenetic protein 2 (BMP2) in bone marrow stromal cells (BMSC) seeded onto a recently developed poly(LLA-co-CL) scaffold.
Methods: Human BMSC were engineered to express VEGFA alone or in combination with BMP2 and seeded onto poly(LLA-co-CL) scaffolds. Changes in angiogenic and osteogenic gene and protein levels were examined by quantitative reverse-transcription polymerase chain reaction (RT-PCR), PCR array, and alkaline phosphatase assay. An in vivo subcutaneous mouse model was used to investigate the effect on angio- and osteogenesis of VEGFA alone or in combination with BMP2, using microcomputed tomography (μCT), histology, immunohistochemistry, and immunofluorescence.
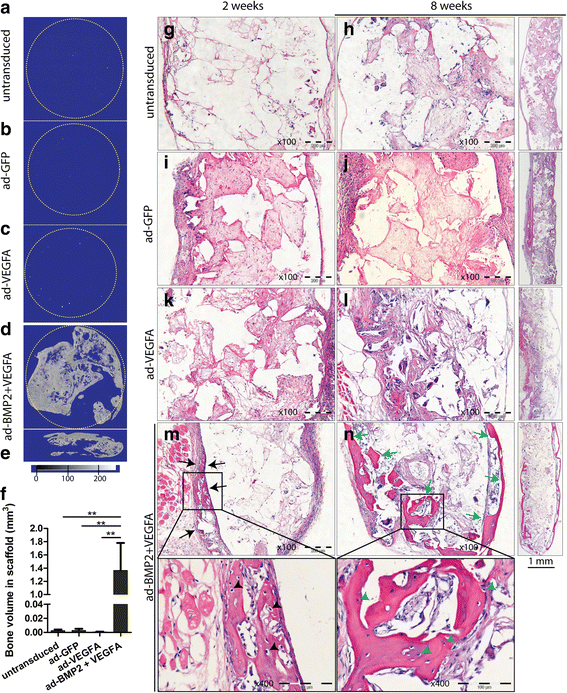
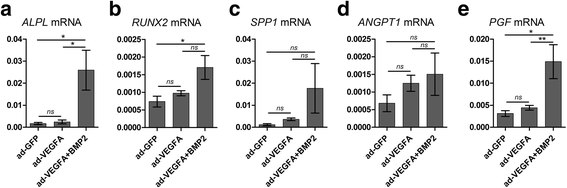
Results: Combined delivery of a lower ratio (1:3) of VEGFA and BMP2 (ad-BMP2 + VEGFA) led to upregulation of osteogenic and angiogenic genes in vitro at 3 and 14 days, compared with mono-delivery of VEGFA (ad-VEGFA) and other controls. In vivo, in a subcutaneous mouse model, both ad-VEGFA and ad-BMP2 + VEGFA scaffold explants exhibited increased angiogenesis at 2 weeks. Enhanced angiogenesis was largely related to the recruitment and differentiation of mouse progenitor cells to the endothelial lineage and, to a lesser extent, to endothelial differentiation of the implanted BMSC. μCT and histological analyses revealed enhanced de novo bone formation only in the ad-BMP2 + VEGFA group, corresponding at the molecular level to the upregulation of genes related to osteogenesis, such as ALPL, RUNX2, and SPP1.
Conclusions: Although BMSC expressing VEGFA alone or in combination with BMP2 significantly induced angiogenesis, VEGFA alone failed to demonstrate osteogenic activity both in vitro and in vivo. These results not only call into question the use of VEGFA alone in bone regeneration, but also highlight the importance in BTE of appropriately formulated combined delivery of VEGFA and BMP2.
Keywords: Angiogenesis; BMP2 and VEGFA; Bone regeneration; Gene delivery; Mesenchymal stem cell; Scaffold.
Conflict of interest statement
Ethics approval and consent to participate
All animal experiments were approved by the Norwegian Animal Research Authority and conducted according to the European Convention for the Protection of Vertebrates used for Scientific Purposes (local approval number 4940). The normal human oral mucosa specimen was collected after informed written patient consent and was approved by the Committee for Medical and Health Research Ethics in West Norway (ref. 2011/1244/REK vest).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Adenoviral Mediated Expression of BMP2 by Bone Marrow Stromal Cells Cultured in 3D Copolymer Scaffolds Enhances Bone Formation.PLoS One. 2016 Jan 25;11(1):e0147507. doi: 10.1371/journal.pone.0147507. eCollection 2016. PLoS One. 2016. PMID: 26808122 Free PMC article.
-
Adenoviral mediated mono delivery of BMP2 is superior to the combined delivery of BMP2 and VEGFA in bone regeneration in a critical-sized rat calvarial bone defect.Bone Rep. 2019 Apr 11;10:100205. doi: 10.1016/j.bonr.2019.100205. eCollection 2019 Jun. Bone Rep. 2019. PMID: 31193299 Free PMC article.
-
In vitro effects of recombinant adenovirus-mediated bone morphogenetic protein 2/vascular endothelial growth factor 165 on osteogenic differentiation of bone marrow mesenchymal stem cells.Artif Cells Nanomed Biotechnol. 2017 Feb;45(1):108-114. doi: 10.3109/21691401.2015.1129629. Epub 2016 Jan 13. Artif Cells Nanomed Biotechnol. 2017. PMID: 26757978
-
Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration.Int J Mol Sci. 2020 May 3;21(9):3242. doi: 10.3390/ijms21093242. Int J Mol Sci. 2020. PMID: 32375269 Free PMC article. Review.
-
Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Oct;103:109761. doi: 10.1016/j.msec.2019.109761. Epub 2019 May 17. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31349418 Review.
Cited by
-
Preclinical Evaluation and Advancements in Vascularized Bone Tissue Engineering.Biomimetics (Basel). 2025 Jun 20;10(7):412. doi: 10.3390/biomimetics10070412. Biomimetics (Basel). 2025. PMID: 40710225 Free PMC article. Review.
-
Angiogenic property of silk fibroin scaffolds with adipose-derived stem cells on chick chorioallantoic membrane.R Soc Open Sci. 2021 Mar 24;8(3):201618. doi: 10.1098/rsos.201618. R Soc Open Sci. 2021. PMID: 33959331 Free PMC article.
-
Extracellular vesicles: From bone development to regenerative orthopedics.Mol Ther. 2023 May 3;31(5):1251-1274. doi: 10.1016/j.ymthe.2023.02.021. Epub 2023 Mar 3. Mol Ther. 2023. PMID: 36869588 Free PMC article. Review.
-
Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials.Pharmaceutics. 2021 Nov 22;13(11):1981. doi: 10.3390/pharmaceutics13111981. Pharmaceutics. 2021. PMID: 34834396 Free PMC article. Review.
-
Accelerated Bone Regeneration by Adrenomedullin 2 Through Improving the Coupling of Osteogenesis and Angiogenesis via β-Catenin Signaling.Front Cell Dev Biol. 2021 Apr 14;9:649277. doi: 10.3389/fcell.2021.649277. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937244 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

