An OTU deubiquitinating enzyme in Eimeria tenella interacts with Eimeria tenella virus RDRP
- PMID: 29386062
- PMCID: PMC5793433
- DOI: 10.1186/s13071-018-2626-x
An OTU deubiquitinating enzyme in Eimeria tenella interacts with Eimeria tenella virus RDRP
Abstract
Background: Chicken coccidiosis, a disease caused by seven species of Eimeria (Apicomplexa: Coccidia), inflicts severe economic losses on the poultry industry. Eimeria tenella is the one of the most virulent species pathogenic to chickens. Many parasitic protozoans are parasitised by double-stranded (ds) RNA viruses, and the influence of protozoan viruses on parasitic protozoans has been extensively reported. E. tenella RNA virus 1 (Etv) was identified in E. tenella, and the complete genome sequence of Etv was analysed. Here, we screened Etv-RNA-dependent RNA polymerase (RDRP)-interacting host protein E. tenella ovarian tumour (OTU) protein-like cysteine protease (Et-OTU) using a yeast two-hybrid system with pGBKT7-RDRP plasmid serving as bait. A previous study demonstrated that Et-OTU could regulate the telomerase activity of E. tenella, indicating that Et-OTU affects E. tenella proliferation. However, whether Etv-RDRP affects the molecular biological characteristics of E. tenella by interacting with OTU remains unclear.
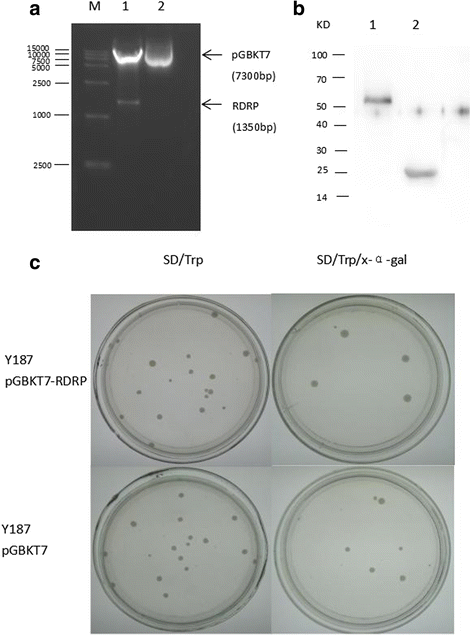
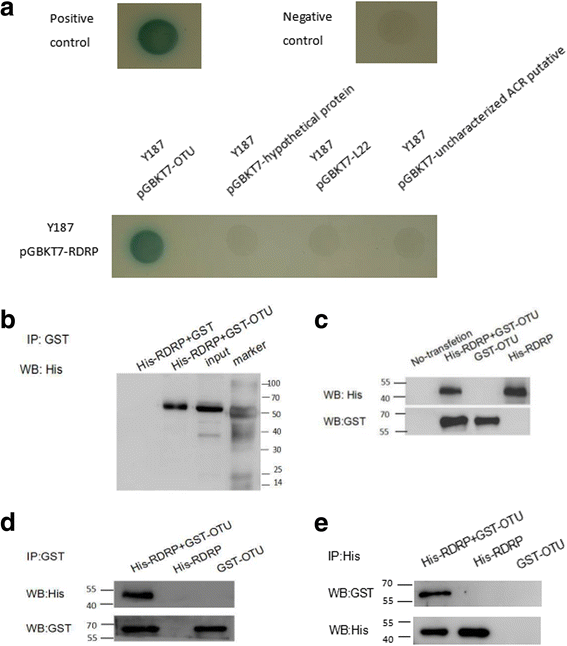
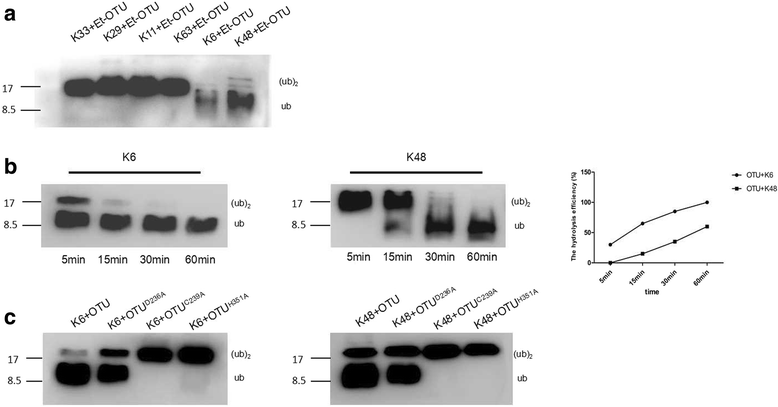
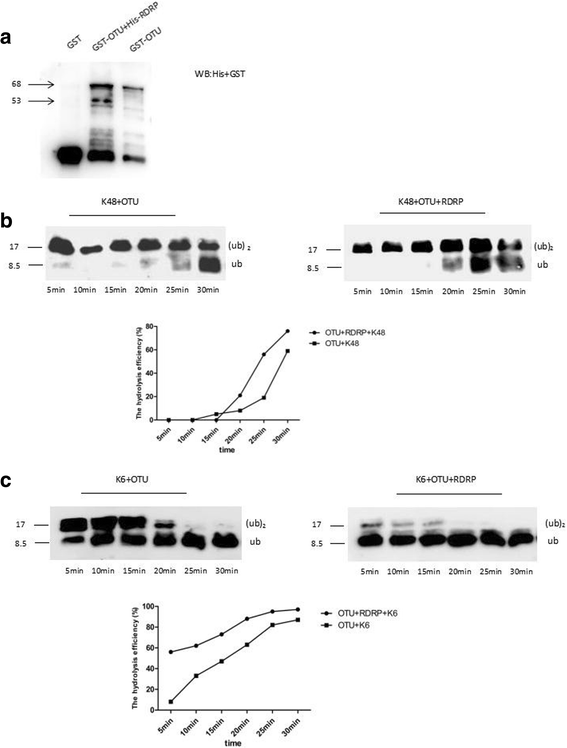
Results: We obtained seven positive clones from the initial screen, and six of the seven preys were identified as false-positives. Finally, we identified an RDRP-associated protein predicted to be an E. tenella OTU protein. A α-galactosidase assay showed that the bait vector did not activate the GAL4 reporter gene, indicating no autoactivation activity from the RDRP bait fusion. Pull-down and co-immunoprecipitation assays verified the interaction between Et-OTU and Etv-RDRP both intracellularly and extracellularly. Additionally, Et-OTU was able to deconjugate K48- and K6-linked di-ubiquitin (di-Ub) chains in vitro but not K63-, K11-, K29-, or K33-linked di-Ub chains. The C239A and H351A mutations eliminated the deubiquitinase (DUB) activity of Et-OTU, whereas the D236A mutation did not. Additionally, when combined with RDRP, the DUB activity of Et-OTU towards K48- and K6-linked chains was significantly enhanced.
Conclusion: Etv-RDRP interacts with Et-OTU both intracellularly and extracellularly. Etv-RDRP enhances the hydrolysis of Et-OTU to K6- or K48-linked ubiquitin chains. This study lays the foundation for further research on the relationship between E. tenella and Etv.
Keywords: Deubiquitinase; Eimeria tenella; Enhanced; Et-OTU; Etv-RDRP; Interaction; Mutation.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
An Eimeria acervulina OTU protease exhibits linkage-specific deubiquitinase activity.Parasitol Res. 2019 Jan;118(1):47-55. doi: 10.1007/s00436-018-6113-2. Epub 2018 Nov 10. Parasitol Res. 2019. PMID: 30415394
-
Screening and characterization of apical membrane antigen 1 interacting proteins in Eimeria tenella.Exp Parasitol. 2016 Nov;170:116-124. doi: 10.1016/j.exppara.2016.09.014. Epub 2016 Sep 28. Exp Parasitol. 2016. PMID: 27693220
-
Virus-like particles in Eimeria tenella are associated with multiple RNA segments.Exp Parasitol. 2011 Mar;127(3):646-50. doi: 10.1016/j.exppara.2010.12.005. Epub 2010 Dec 22. Exp Parasitol. 2011. PMID: 21184756
-
Plant deubiquitinases: from structure and activity to biological functions.Plant Cell Rep. 2023 Mar;42(3):469-486. doi: 10.1007/s00299-022-02962-y. Epub 2022 Dec 26. Plant Cell Rep. 2023. PMID: 36567335 Review.
-
Role of Virally-Encoded Deubiquitinating Enzymes in Regulation of the Virus Life Cycle.Int J Mol Sci. 2021 Apr 23;22(9):4438. doi: 10.3390/ijms22094438. Int J Mol Sci. 2021. PMID: 33922750 Free PMC article. Review.
Cited by
-
The emerging role of Deubiquitinases (DUBs) in parasites: A foresight review.Front Cell Infect Microbiol. 2022 Sep 27;12:985178. doi: 10.3389/fcimb.2022.985178. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36237424 Free PMC article. Review.
-
Identification and Protective Efficacy of Eimeria tenella Rhoptry Kinase Family Protein 17.Animals (Basel). 2022 Feb 23;12(5):556. doi: 10.3390/ani12050556. Animals (Basel). 2022. PMID: 35268126 Free PMC article.
-
A potential role for Giardia chaperone protein GdDnaJ in regulating Giardia proliferation and Giardiavirus replication.Parasit Vectors. 2023 May 25;16(1):168. doi: 10.1186/s13071-023-05787-0. Parasit Vectors. 2023. PMID: 37226181 Free PMC article.
-
Characterisation of the OTU domain deubiquitinase complement of Toxoplasma gondii.Life Sci Alliance. 2023 Mar 23;6(6):e202201710. doi: 10.26508/lsa.202201710. Print 2023 Jun. Life Sci Alliance. 2023. PMID: 36958824 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

