Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice
- PMID: 29386068
- PMCID: PMC5793353
- DOI: 10.1186/s13287-017-0745-5
Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice
Retraction in
-
Retraction Note: Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice.Stem Cell Res Ther. 2022 Oct 13;13(1):504. doi: 10.1186/s13287-022-03183-6. Stem Cell Res Ther. 2022. PMID: 36224609 Free PMC article. No abstract available.
Abstract
Background: This study was performed to determine the effects of human placenta mesenchymal stem cell (hPMSC) transplantation on granulosa cell apoptosis and anti-Müllerian hormone (AMH) and follicle-stimulating hormone receptor (FSHR) expression in autoimmune drug-induced premature ovarian failure (POF) mice. The aim of this research is to investigate the mechanisms of hPMSCs on ovarian reserve capacity.
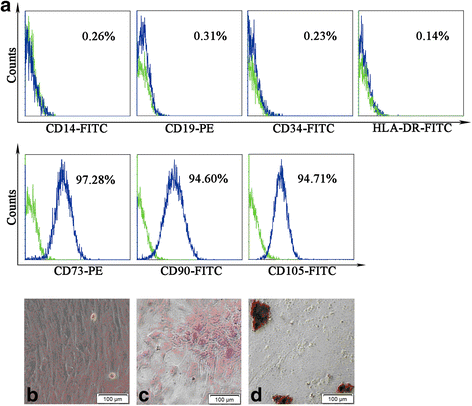
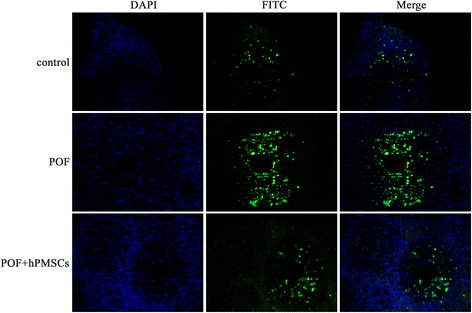
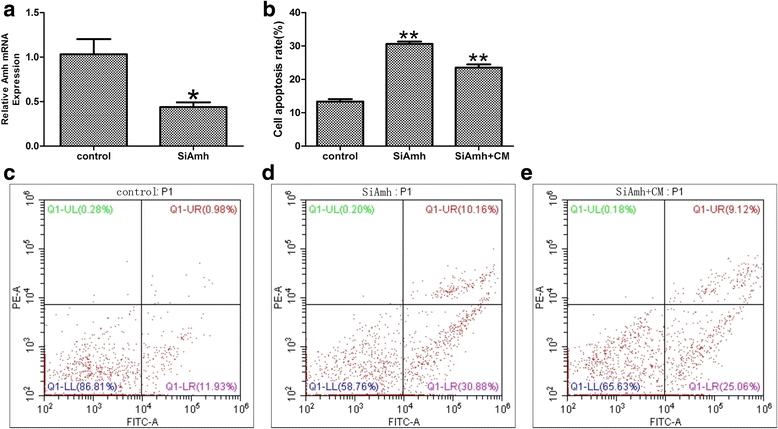
Methods: The POF mice model was established by injection of zona pellucida 3 peptide (pZP3). hPMSC transplantation was conducted by intravenous injection into mice following pZP3 treatment. The follicle number was examined by histopathology. The serum levels of FSH, LH, E2, AMH and anti-zona pellucida antibody (AzpAb) were measured by enzyme-linked immunosorbent assay. AMH and FSHR expression in the ovary was analyzed by immunohistochemistry and western blot analysis. Granulosa cell apoptosis of the ovaries was examined by In Situ Cell Death Detection Kit. Granulosa cells were isolated and treated with SiAmh interference and hPMSC supernatant to observe the effects of AMH expression on granulosa cell apoptosis in vitro.
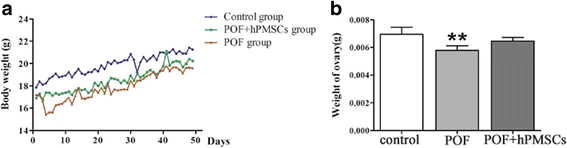
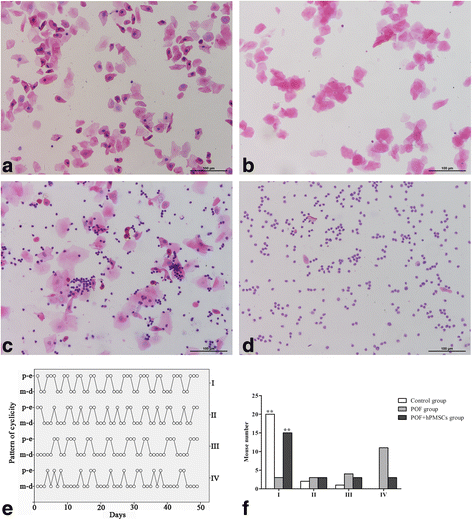
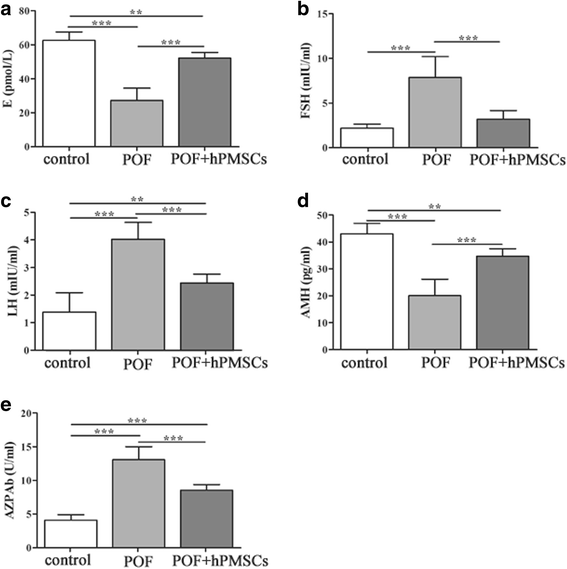
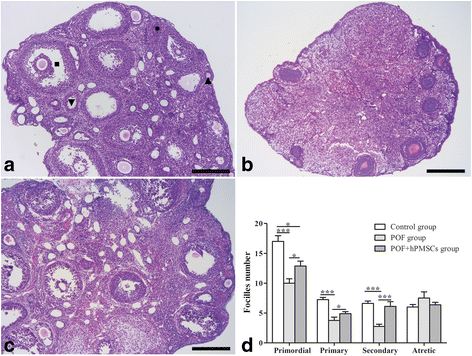
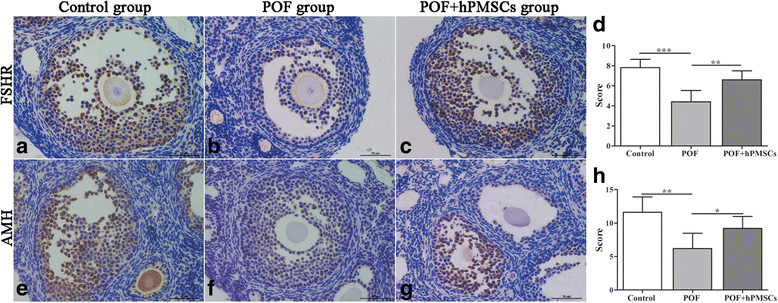
Results: The results showed that hPMSC transplantation can significantly recover the estrus cycle in the POF group. Morphological staining showed that the basal follicles and sinus follicles after hPMSC transplantation were higher in POF mice than in those without treatment, and the follicle number was significantly decreased with atresia. The serum levels of FSH, LH and AzpAb in the hPMSC transplantation group were reduced considerably, but the E2 and AMH levels were significantly increased. After hPMSC transplantation, the AMH and FSHR expression in ovarian tissue was significantly higher than in the POF group as determined by immunochemistry and western blot analysis. The FSHR expression was shown in granulosa cells only, and FSHR expression increases with AMH expressed in the ovary; granulosa cell apoptosis was decreased following hPMSC transplantation. The same results were observed from the in-vitro study.
Conclusions: hPMSC transplantation can significantly improve the serum levels of high gonadotropin and low estrogen of POF mice, promote follicular development, inhibit excessive follicular atresia and granulosa cell apoptosis, and improve the ovarian reserve capacity. The mechanism may be achieved by increasing the expression of AMH and FSHR in ovaries.
Keywords: Anti-Müllerian hormone; Apoptosis; Follicle stimulating hormone receptor; Granulosa cells; Mesenchymal stem cell; Placenta; Premature ovarian failure.
Conflict of interest statement
Ethics approval
All animal care and experimental procedures were performed according to the guide for the Care and Use of Laboratory Animals established by the National Research Council (National Academy Press Washington, DC, 1996), and were approved by the Institutional Animal Care and Use Committee of Binzhou Medical University. The use of human tissue was approved by the Institutional Ethics Committee.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

