Distinction between serological responses following tick-borne encephalitis virus (TBEV) infection vs vaccination, Sweden 2017
- PMID: 29386094
- PMCID: PMC5792698
- DOI: 10.2807/1560-7917.ES.2018.23.3.17-00838
Distinction between serological responses following tick-borne encephalitis virus (TBEV) infection vs vaccination, Sweden 2017
Abstract
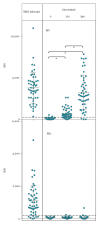
Tick-borne encephalitis virus (TBEV) is an important European vaccine-preventable pathogen. Discrimination of vaccine-induced antibodies from those elicited by infection is important. We studied anti-TBEV IgM/IgG responses, including avidity and neutralisation, by multiplex serology in 50 TBEV patients and 50 TBEV vaccinees. Infection induced antibodies reactive to both whole virus (WV) and non-structural protein 1 (NS1) in 48 clinical cases, whereas 47 TBEV vaccinees had WV, but not NS1 antibodies, enabling efficient discrimination of infection/vaccination.
Keywords: Tick-borne encephalitis (TBE); diagnostics; vaccine-preventable diseases; vaccines and immunisation; viral encephalitis; zoonotic infections.
Conflict of interest statement
Figures

Similar articles
-
Confirmed exposure to tick-borne encephalitis virus and probable human cases of tick-borne encephalitis in Central/Northern Anatolia, Turkey.Zoonoses Public Health. 2011 May;58(3):220-7. doi: 10.1111/j.1863-2378.2010.01342.x. Zoonoses Public Health. 2011. PMID: 20604912
-
No detection of tick-borne encephalitis virus RNA in blood, urine or saliva of hospitalised immunocompetent tick-borne encephalitis patients.PLoS One. 2024 Jun 24;19(6):e0305603. doi: 10.1371/journal.pone.0305603. eCollection 2024. PLoS One. 2024. PMID: 38913668 Free PMC article.
-
Tick-Borne Encephalitis Virus Surveillance-Vaccination- and Infection-Induced Seroprevalences in Western Austria 2024.J Med Virol. 2024 Dec;96(12):e70109. doi: 10.1002/jmv.70109. J Med Virol. 2024. PMID: 39660431
-
[Tick-borne encephalitis].Nervenarzt. 2016 Jun;87(6):667-80. doi: 10.1007/s00115-016-0134-9. Nervenarzt. 2016. PMID: 27225401 Review. German.
-
Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines.Antiviral Res. 2019 Apr;164:23-51. doi: 10.1016/j.antiviral.2019.01.014. Epub 2019 Jan 31. Antiviral Res. 2019. PMID: 30710567 Review.
Cited by
-
Tick-borne encephalitis vaccine breakthrough infections induce aberrant T cell and antibody responses to non-structural proteins.NPJ Vaccines. 2024 Aug 7;9(1):141. doi: 10.1038/s41541-024-00936-7. NPJ Vaccines. 2024. PMID: 39112523 Free PMC article.
-
Tick-Borne Encephalitis Virus Vaccines Contain Non-Structural Protein 1 Antigen and may Elicit NS1-Specific Antibody Responses in Vaccinated Individuals.Vaccines (Basel). 2020 Feb 12;8(1):81. doi: 10.3390/vaccines8010081. Vaccines (Basel). 2020. PMID: 32059489 Free PMC article.
-
Low-Energy Electron Irradiation of Tick-Borne Encephalitis Virus Provides a Protective Inactivated Vaccine.Front Immunol. 2022 Mar 7;13:825702. doi: 10.3389/fimmu.2022.825702. eCollection 2022. Front Immunol. 2022. PMID: 35340807 Free PMC article.
-
Antibody responses to tick-borne encephalitis virus non-structural protein 1 and whole virus antigen-a new tool in the assessment of suspected vaccine failure patients.Infect Ecol Epidemiol. 2019 Dec 3;9(1):1696132. doi: 10.1080/20008686.2019.1696132. eCollection 2019. Infect Ecol Epidemiol. 2019. PMID: 31839903 Free PMC article.
-
Broad and potent neutralizing human antibodies to tick-borne flaviviruses protect mice from disease.J Exp Med. 2021 May 3;218(5):e20210236. doi: 10.1084/jem.20210236. J Exp Med. 2021. PMID: 33831141 Free PMC article.
References
-
- Rönnberg B, Gustafsson Å, Vapalahti O, Emmerich P, Lundkvist Å, Schmidt-Chanasit J, et al. Compensating for cross-reactions using avidity and computation in a suspension multiplex immunoassay for serotyping of Zika versus other flavivirus infections. Med Microbiol Immunol (Berl). 2017;206(5):383-401. 10.1007/s00430-017-0517-y - DOI - PMC - PubMed
-
- Dobler G, Tkachev S. General epidemiology of tick-borne encephalitis. In: Dobler G, Erber W, Schmitt HJ. TBE-The Book. Singapore: Global Health Press; 2017. ISBN: 978-981-1903-3. Available from: https://id-ea.org/tbe
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
