Improving T-cell expansion and function for adoptive T-cell therapy using ex vivo treatment with PI3Kδ inhibitors and VIP antagonists
- PMID: 29386194
- PMCID: PMC5812323
- DOI: 10.1182/bloodadvances.2017011254
Improving T-cell expansion and function for adoptive T-cell therapy using ex vivo treatment with PI3Kδ inhibitors and VIP antagonists
Abstract
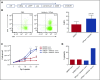
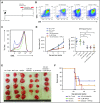
Adoptive therapy with ex vivo-expanded genetically modified antigen-specific T cells can induce remissions in patients with relapsed/refractory cancer. The clinical success of this therapy depends upon efficient transduction and expansion of T cells ex vivo and their homing, persistence and cytotoxicity following reinfusion. Lower rates of ex vivo expansion and clinical response using anti-CD19 chimeric antigen receptor (CAR) T cells have been seen in heavily pretreated lymphoma patients compared with B-cell acute lymphoblastic leukemia patients and motivate the development of novel strategies to enhance ex vivo T cell expansion and their persistence in vivo. We demonstrate that inhibition of phosphatidylinositol 3-kinase δ (PI3Kδ) and antagonism of vasoactive intestinal peptide (VIP) signaling partially inhibits the terminal differentiation of T cells during anti-CD3/CD28 bead-mediated expansion (mean, 54.4% CD27+CD28+ T cells vs 27.4% in control cultures; P < .05). This strategy results in a mean of 83.7% more T cells cultured from lymphoma patients in the presence of PI3Kδ and VIP antagonists, increased survival of human T cells from a lymphoma patient in a murine xenograft model, enhanced cytotoxic activity of antigen-specific human CAR T cells and murine T cells against lymphoma, and increased transduction and expansion of anti-CD5 human CAR T cells. PI3Kδ and VIP antagonist-expanded T cells from lymphoma patients show reduced terminal differentiation, enhanced polyfunctional cytokine expression, and preservation of costimulatory molecule expression. Taken together, synergistic blockade of these pathways is an attractive strategy to enhance the expansion and functional capacity of ex vivo-expanded cancer-specific T cells.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

