Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia
- PMID: 29386195
- PMCID: PMC5812332
- DOI: 10.1182/bloodadvances.2017014175
Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia
Abstract
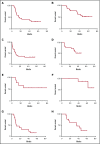
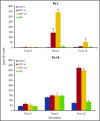
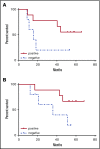
A National Cancer Institute consensus study on prioritization of cancer antigens ranked the Wilms tumor 1 (WT1) protein as the top immunotherapy target in cancer. We previously reported a pilot study of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia (AML) patients. We have now conducted a phase 2 study investigating this vaccine in adults with AML in first complete remission (CR1). Patients received 6 vaccinations administered over 10 weeks with the potential to receive 6 additional monthly doses if they remained in CR1. Immune responses (IRs) were evaluated after the 6th and 12th vaccinations by CD4+ T-cell proliferation, CD8+ T-cell interferon-γ secretion (enzyme-linked immunospot), or the CD8-relevant WT1 peptide major histocompatibility complex tetramer assay (HLA-A*02 patients only). Twenty-two patients (7 males; median age, 64 years) were treated. Fourteen patients (64%) completed ≥6 vaccinations, and 9 (41%) received all 12 vaccine doses. Fifteen patients (68%) relapsed, and 10 (46%) died. The vaccine was well tolerated, with the most common toxicities being grade 1/2 injection site reactions (46%), fatigue (32%), and skin induration (32%). Median disease-free survival from CR1 was 16.9 months, whereas the overall survival from diagnosis has not yet been reached but is estimated to be ≥67.6 months. Nine of 14 tested patients (64%) had an IR in ≥1 assay (CD4 or CD8). These results indicated that the WT1 vaccine was well tolerated, stimulated a specific IR, and was associated with survival in excess of 5 years in this cohort of patients. This trial was registered at www.clinicaltrials.gov as #NCT01266083.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.G.M. receives research funding from Sellas Life Sciences. L.K. and N.S. are employees of Sellas Life Sciences Group and receive salary and stock options as compensation for their employment. D.A.S. is inventor of the vaccine and a consultant to Sellas. The remaining authors declare no competing financial interests.
Figures



References
-
- Khalil DN, Budhu S, Gasmi B, et al. . The new era of cancer immunotherapy: manipulating T-cell activity to overcome malignancy. Adv Cancer Res. 2015;128:1-68. - PubMed
-
- Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol. 2008;142(6):877-888. - PubMed
-
- Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861-3868. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

