Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations
- PMID: 29386298
- PMCID: PMC5909767
- DOI: 10.1136/thoraxjnl-2017-210408
Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations
Abstract
Background: Alterations in the composition of the lung microbiome associated with adverse clinical outcomes, known as dysbiosis, have been implicated with disease severity and exacerbations in COPD.
Objective: To characterise longitudinal changes in the lung microbiome in the AERIS study (Acute Exacerbation and Respiratory InfectionS in COPD) and their relationship with associated COPD outcomes.
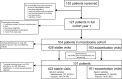
Methods: We surveyed 584 sputum samples from 101 patients with COPD to analyse the lung microbiome at both stable and exacerbation time points over 1 year using high-throughput sequencing of the 16S ribosomal RNA gene. We incorporated additional lung microbiology, blood markers and in-depth clinical assessments to classify COPD phenotypes.
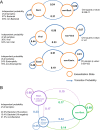
Results: The stability of the lung microbiome over time was more likely to be decreased in exacerbations and within individuals with higher exacerbation frequencies. Analysis of exacerbation phenotypes using a Markov chain model revealed that bacterial and eosinophilic exacerbations were more likely to be repeated in subsequent exacerbations within a subject, whereas viral exacerbations were not more likely to be repeated. We also confirmed the association of bacterial genera, including Haemophilus and Moraxella, with disease severity, exacerbation events and bronchiectasis.
Conclusions: Subtypes of COPD have distinct bacterial compositions and stabilities over time. Some exacerbation subtypes have non-random probabilities of repeating those subtypes in the future. This study provides insights pertaining to the identification of bacterial targets in the lung and biomarkers to classify COPD subtypes and to determine appropriate treatments for the patient.
Trial registration number: Results, NCT01360398.
Keywords: Copd exacerbations; Copd ÀÜ mechanisms; respiratory infection.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: TMW has received reimbursement for travel and meeting attendance from Boehringer Ingelheim and AstraZeneca, outside of the submitted work. SC received a grant from Pfizer, outside of the submitted work. KJS received grants from Asthma UK (08/026) and BMA HC Roscoe Award, outside of the submitted work, and he has a patent PCT/GB2010/050821 ’Ex Vivo Modelling of Therapeutic Interventions' pending. BEM, CL, DFS, DM, GS, J-MD, JRB, ND, MM-S, RS, RT-S, SVH and VW are employees of the GSK group of companies. RP was an employee of the GSK group of companies at the time the study was conducted. BEM, JRB, J-MD, ND, RT-S and VW own shares/restricted shares in the GSK group of companies. KJS, VK, KO, ACT, SC and TMW received an institutional grant from the GSK group of companies to conduct this study.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
