Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration
- PMID: 29386381
- PMCID: PMC5816207
- DOI: 10.1073/pnas.1720553115
Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration
Abstract
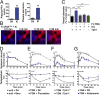
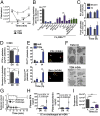
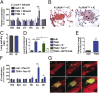
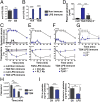
There is a growing appreciation for the contribution of platelets to immunity; however, our knowledge mostly relies on platelet functions associated with vascular injury and the prevention of bleeding. Circulating immune complexes (ICs) contribute to both chronic and acute inflammation in a multitude of clinical conditions. Herein, we scrutinized platelet responses to systemic ICs in the absence of tissue and endothelial wall injury. Platelet activation by circulating ICs through a mechanism requiring expression of platelet Fcγ receptor IIA resulted in the induction of systemic shock. IC-driven shock was dependent on release of serotonin from platelet-dense granules secondary to platelet outside-in signaling by αIIbβ3 and its ligand fibrinogen. While activated platelets sequestered in the lungs and leaky vasculature of the blood-brain barrier, platelets also sequestered in the absence of shock in mice lacking peripheral serotonin. Unexpectedly, platelets returned to the blood circulation with emptied granules and were thereby ineffective at promoting subsequent systemic shock, although they still underwent sequestration. We propose that in response to circulating ICs, platelets are a crucial mediator of the inflammatory response highly relevant to sepsis, viremia, and anaphylaxis. In addition, platelets recirculate after degranulation and sequestration, demonstrating that in adaptive immunity implicating antibody responses, activated platelets are longer lived than anticipated and may explain platelet count fluctuations in IC-driven diseases.
Keywords: Fc receptor; immune complexes; platelets; serotonin; thrombocytopenia.
Conflict of interest statement
Conflict of interest statement: J.E.I. has a financial interest in and is a founder of Platelet BioGenesis, a company that aims to produce donor-independent human platelets from human-induced pluripotent stem cells at scale. J.E.I. is an inventor on this patent. The interests of J.E.I. were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Figures

References
-
- Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. - PubMed
-
- Kapur R, Zufferey A, Boilard E, Semple JW. Nouvelle cuisine: Platelets served with inflammation. J Immunol. 2015;194:5579–5587. - PubMed
-
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

