Identification and functional characterization of arginine vasopressin receptor 1A : atypical chemokine receptor 3 heteromers in vascular smooth muscle
- PMID: 29386406
- PMCID: PMC5795052
- DOI: 10.1098/rsob.170207
Identification and functional characterization of arginine vasopressin receptor 1A : atypical chemokine receptor 3 heteromers in vascular smooth muscle
Abstract
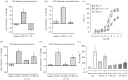
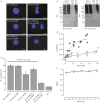
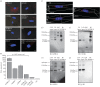
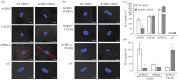
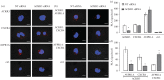
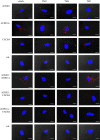
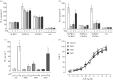
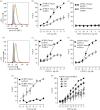
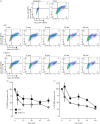
Recent observations suggest that atypical chemokine receptor (ACKR)3 and chemokine (C-X-C motif) receptor (CXCR)4 regulate human vascular smooth muscle function through hetero-oligomerization with α1-adrenoceptors. Here, we show that ACKR3 also regulates arginine vasopressin receptor (AVPR)1A. We observed that ACKR3 agonists inhibit arginine vasopressin (aVP)-induced inositol trisphosphate (IP3) production in human vascular smooth muscle cells (hVSMCs) and antagonize aVP-mediated constriction of isolated arteries. Proximity ligation assays, co-immunoprecipitation and bioluminescence resonance energy transfer experiments suggested that recombinant and endogenous ACKR3 and AVPR1A interact on the cell surface. Interference with ACKR3 : AVPR1A heteromerization using siRNA and peptide analogues of transmembrane domains of ACKR3 abolished aVP-induced IP3 production. aVP stimulation resulted in β-arrestin 2 recruitment to AVPR1A and ACKR3. While ACKR3 activation failed to cross-recruit β-arrestin 2 to AVPR1A, the presence of ACKR3 reduced the efficacy of aVP-induced β-arrestin 2 recruitment to AVPR1A. AVPR1A and ACKR3 co-internalized upon agonist stimulation in hVSMC. These data suggest that AVPR1A : ACKR3 heteromers are constitutively expressed in hVSMC, provide insights into molecular events at the heteromeric receptor complex, and offer a mechanistic basis for interactions between the innate immune and vasoactive neurohormonal systems. Our findings suggest that ACKR3 is a regulator of vascular smooth muscle function and a possible drug target in diseases associated with impaired vascular reactivity.
Keywords: CXCL11; CXCL12; arginine vasopressin; ubiquitin; vasoconstriction; vasopressor.
© 2018 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
-
- Gambaryan N, Perros F, Montani D, Cohen-Kaminsky S, Mazmanian M, Renaud JF, Simonneau G, Lombet A, Humbert M. 2011. Targeting of c-kit+ haematopoietic progenitor cells prevents hypoxic pulmonary hypertension. Eur. Respir. J. 37, 1392–1399. (doi:10.1183/09031936.00045710) - DOI - PubMed
-
- Yu L, Hales CA. 2011. Effect of chemokine receptor CXCR4 on hypoxia-induced pulmonary hypertension and vascular remodeling in rats. Respir. Res. 12, 21 (doi:10.1186/1465-9921-12-21) - DOI - PMC - PubMed
-
- Chu PY, Zatta A, Kiriazis H, Chin-Dusting J, Du XJ, Marshall T, Kaye DM. 2011. CXCR4 antagonism attenuates the cardiorenal consequences of mineralocorticoid excess. Circ. Heart Fail. 4, 651–658. (doi:10.1161/CIRCHEARTFAILURE.110.960831) - DOI - PubMed
-
- Sartina E, et al. 2012. Antagonism of CXCR7 attenuates chronic hypoxia-induced pulmonary hypertension. Pediatr. Res. 71, 682–688. (doi:10.1038/pr.2012.30) - DOI - PubMed
-
- Bach HH, Wong YM, LaPorte HM, Gamelli RL, Majetschak M. 2016. Pharmacological targeting of chemokine (C-X-C motif) receptor 4 in porcine polytrauma and hemorrhage models. J. Trauma Acute Care Surg. 80, 102–110. (doi:10.1097/TA.0000000000000865) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

