Bortezomib inhibits proliferation, migration, and TGF-β1-induced epithelial-mesenchymal transition of RPE cells
- PMID: 29386876
- PMCID: PMC5757857
Bortezomib inhibits proliferation, migration, and TGF-β1-induced epithelial-mesenchymal transition of RPE cells
Abstract
Purpose: Nuclear factor kappa B (NF-κB) plays an important role in the epithelial-mesenchymal transition (EMT) of RPE cells. We investigated the effects of a proteasome inhibitor, bortezomib, on the EMT in RPE cells. In addition, we assessed the influence of bortezomib on regulation of the NF-κB pathway during this process.
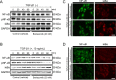
Methods: After treatment with various concentrations of bortezomib, cell viability was analyzed with the water-soluble tetrazolium salt-8 assay, cell-cycle regulation was evaluated with flow cytometry, and cell migration was monitored with in vitro wound healing and Transwell migration assays. To induce fibroblastoid transformation, the RPE cells were treated with recombinant human transforming growth factor (TGF)-β1 (10 ng/ml), and western blot and immunocytochemical analyses were performed to evaluate altered expression of EMT markers after treatment with bortezomib. To verify the effect of bortezomib on shrinkage by myofibroblastic transformation, a contraction assay of the RPE-collagen gel lattice was performed.
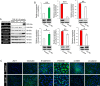
Results: Treatment with bortezomib decreased RPE viability in a dose-dependent manner, and flow cytometry revealed that these effects were due to arrest of the G2/M phase cell-cycle. In the in vitro wound healing and Transwell migration assays, treatment with 20 nM bortezomib significantly impeded RPE migration. Treatment with bortezomib also significantly inhibited TGF-β1-induced transdifferentiation of the RPE cells. The effects on proliferation, migration, and the EMT were mediated by regulation of the NF-κB signaling pathway. In addition, bortezomib inhibited contraction of the RPE-collagen gel lattices.
Conclusions: Bortezomib inhibits myofibroblastic transformation of RPE cells by downregulating NF-κB expression and prevents contraction of the RPE-collagen gel matrix. Thus, bortezomib represents a candidate putative therapeutic agent for management of retinal fibrotic diseases.
Figures



Similar articles
-
TGF-β1 induced transdifferentiation of rpe cells is mediated by TAK1.PLoS One. 2015 Apr 7;10(4):e0122229. doi: 10.1371/journal.pone.0122229. eCollection 2015. PLoS One. 2015. PMID: 25849436 Free PMC article.
-
Quercetin inhibits transforming growth factor β1-induced epithelial-mesenchymal transition in human retinal pigment epithelial cells via the Smad pathway.Drug Des Devel Ther. 2018 Dec 6;12:4149-4161. doi: 10.2147/DDDT.S185618. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30584279 Free PMC article.
-
TGF-β2 promotes RPE cell invasion into a collagen gel by mediating urokinase-type plasminogen activator (uPA) expression.Exp Eye Res. 2013 Oct;115:13-21. doi: 10.1016/j.exer.2013.06.020. Epub 2013 Jun 28. Exp Eye Res. 2013. PMID: 23810810
-
Epithelial-Mesenchymal Transition Induced in Cancer Cells by Adhesion to Type I Collagen.Int J Mol Sci. 2022 Dec 22;24(1):198. doi: 10.3390/ijms24010198. Int J Mol Sci. 2022. PMID: 36613638 Free PMC article. Review.
-
Rebooting the collagen gel: Artificial hydrogels for the study of epithelial mesenchymal transformation.Dev Dyn. 2018 Mar;247(3):332-339. doi: 10.1002/dvdy.24560. Epub 2017 Sep 5. Dev Dyn. 2018. PMID: 28786157 Review.
Cited by
-
TGF-β Superfamily Signaling in the Eye: Implications for Ocular Pathologies.Cells. 2022 Jul 29;11(15):2336. doi: 10.3390/cells11152336. Cells. 2022. PMID: 35954181 Free PMC article. Review.
-
RPE epithelial-mesenchymal transition plays a critical role in the pathogenesis of proliferative vitreoretinopathy.Ann Transl Med. 2020 Mar;8(6):263. doi: 10.21037/atm.2020.03.86. Ann Transl Med. 2020. PMID: 32355707 Free PMC article. No abstract available.
-
Transforming growth factor-β in tumour development.Front Mol Biosci. 2022 Oct 4;9:991612. doi: 10.3389/fmolb.2022.991612. eCollection 2022. Front Mol Biosci. 2022. PMID: 36267157 Free PMC article. Review.
-
Proteasome inhibitor bortezomib prevents proliferation and migration of pulmonary arterial smooth muscle cells.Kaohsiung J Med Sci. 2024 Jun;40(6):542-552. doi: 10.1002/kjm2.12835. Epub 2024 Apr 29. Kaohsiung J Med Sci. 2024. PMID: 38682650 Free PMC article.
-
GRK5-mediated inflammation and fibrosis exert cardioprotective effects during the acute phase of myocardial infarction.FEBS Open Bio. 2023 Feb;13(2):380-391. doi: 10.1002/2211-5463.13551. Epub 2023 Jan 20. FEBS Open Bio. 2023. PMID: 36633120 Free PMC article.
References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. - PubMed
-
- Birchmeier C, Birchmeier W. Molecular aspects of mesenchymal-epithelial interactions. Annu Rev Cell Biol. 1993;9:511–40. - PubMed
-
- Guarino M. Epithelial-to-mesenchymal change of differentiation. From embryogenetic mechanism to pathological patterns. Histol Histopathol. 1995;10:171–84. - PubMed
-
- Pagan R, Martin I, Alonso A, Llobera M, Vilaro S. Vimentin filaments follow the preexisting cytokeratin network during epithelial-mesenchymal transition of cultured neonatal rat hepatocytes. Exp Cell Res. 1996;222:333–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

