Expression patterns of zebrafish nocturnin genes and the transcriptional activity of the frog nocturnin promoter in zebrafish rod photoreceptors
- PMID: 29386877
- PMCID: PMC5757853
Expression patterns of zebrafish nocturnin genes and the transcriptional activity of the frog nocturnin promoter in zebrafish rod photoreceptors
Abstract
Purpose: Daily modulation of gene expression is critical for the circadian rhythms of many organisms. One of the modulating mechanisms is based on nocturnin, a deadenylase that degrades mRNA in a circadian fashion. The nocturnin genes are expressed broadly, but their tissue expression patterns differ between mice and the frog Xenopus laevis; this difference suggests that the extent of the regulation of nocturin gene expression varies among species. In this study, we set out to characterize the expression patterns of two zebrafish nocturnin genes; in addition, we asked whether a frog nocturnin promoter has transcriptional activity in zebrafish.
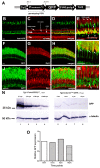
Methods: We used reverse transcription (RT)-PCR, quantitative real-time PCR (qRT-PCR), and rapid amplification of cDNA ends (RACE) analysis to determine whether the nocturnin-a and nocturnin-b genes are expressed in the eye, in situ hybridization to determine the cellular expression pattern of the nocturnin-b gene in the retina, and confocal microscopy to determine the protein expression pattern of the transgenic reporter green fluorescent protein (GFP) driven by the frog nocturnin promoter.
Results: We found that the amino acid sequences of zebrafish nocturnin-a and nocturnin-b are highly similar to those of frog, mouse, and human nocturnin homologs. Only nocturnin-b is expressed in the eye. Within the retina, nocturnin-b mRNA was expressed at higher levels in the retinal photoreceptors layer than in other cellular layers. This expression pattern echoes the restricted photoreceptor expression of nocturnin in the frog. We also found that the frog nocturnin promoter can be specifically activated in zebrafish rod photoreceptors.
Conclusions: The high level of similarities in amino acid sequences of human, mouse, frog, and zebrafish nocturnin homologs suggest these proteins maintain a conserved deadenylation function that is important for regulating retinal circadian rhythmicity. The rod-specific transcriptional activity of the frog nocturnin promoter makes it a useful tool to drive moderate and rod-specific transgenic expression in zebrafish. The results of this study lay the groundwork to study nocturnin-based circadian biology of the zebrafish retina.
Figures


Similar articles
-
Nocturnin, a deadenylase in Xenopus laevis retina: a mechanism for posttranscriptional control of circadian-related mRNA.Curr Biol. 2003 Feb 4;13(3):189-98. doi: 10.1016/s0960-9822(03)00014-9. Curr Biol. 2003. PMID: 12573214
-
A novel promoter element, photoreceptor conserved element II, directs photoreceptor-specific expression of nocturnin in Xenopus laevis.J Biol Chem. 2001 May 4;276(18):15146-54. doi: 10.1074/jbc.M009970200. Epub 2001 Jan 16. J Biol Chem. 2001. PMID: 11278588
-
Microarray analysis of XOPS-mCFP zebrafish retina identifies genes associated with rod photoreceptor degeneration and regeneration.Invest Ophthalmol Vis Sci. 2011 Apr 6;52(5):2255-66. doi: 10.1167/iovs.10-6022. Invest Ophthalmol Vis Sci. 2011. PMID: 21217106 Free PMC article.
-
Molecular control of Xenopus retinal circadian rhythms.J Neuroendocrinol. 2003 Apr;15(4):350-4. doi: 10.1046/j.1365-2826.2003.00999.x. J Neuroendocrinol. 2003. PMID: 12622833 Review.
-
Nocturnin: at the crossroads of clocks and metabolism.Trends Endocrinol Metab. 2012 Jul;23(7):326-33. doi: 10.1016/j.tem.2012.03.007. Epub 2012 May 17. Trends Endocrinol Metab. 2012. PMID: 22608110 Free PMC article. Review.
Cited by
-
Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression.Int J Mol Sci. 2023 Dec 19;25(1):54. doi: 10.3390/ijms25010054. Int J Mol Sci. 2023. PMID: 38203224 Free PMC article.
-
Regulatory roles of vertebrate Nocturnin: insights and remaining mysteries.RNA Biol. 2018;15(10):1255-1267. doi: 10.1080/15476286.2018.1526541. Epub 2018 Oct 9. RNA Biol. 2018. PMID: 30257600 Free PMC article.
-
Neutrophils facilitate the epicardial regenerative response after zebrafish heart injury.Dev Biol. 2024 Apr;508:93-106. doi: 10.1016/j.ydbio.2024.01.011. Epub 2024 Jan 28. Dev Biol. 2024. PMID: 38286185 Free PMC article.
References
-
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. - PubMed
-
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. - PubMed
-
- Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–61. - PubMed
-
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

