Aflibercept for clinically significant diabetic macular edema: 12-month results in daily clinical practice
- PMID: 29386883
- PMCID: PMC5764298
- DOI: 10.2147/OPTH.S154421
Aflibercept for clinically significant diabetic macular edema: 12-month results in daily clinical practice
Abstract
Purpose: To assess the effectiveness and safety of intravitreal aflibercept in clinically significant diabetic macular edema (DME) in daily clinical practice.
Methods: Prospective, open-label, single-center study. Anti-vascular endothelial growth factor naïve patients with clinically significant DME received intravitreal injections of aflibercept 2 mg, five monthly doses followed by a fixed schedule every 2 months for 12 months. The mean change in best-corrected visual acuity (BCVA) (Early Treatment Diabetic Retinopathy Study [ETDRS] letters) was the primary outcome.
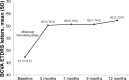
Results: The mean BCVA improved significantly as compared with baseline at 12 months of treatment (47.3 [14.2] vs 62.2 [13.9] ETDRS letters, P<0.001). Significant improvement in BCVA was already observed at visit 2 after the loading doses of aflibercept. At 12 months, gains in ETDRS letters were documented in all eyes (100%), with gains ≥10 letters in 89.6%, ≥15 letters in 65.5%, and ≥20 letters in 6.9% (n=2). A significant reduction in central macular thickness from a mean of 460.5 (11.8) μm at baseline to 229.0 (43.8) μm at 12 months (P<0.001) was observed. Significant reductions of central macular thickness were already observed after the loading doses and continued lowering throughout the study period. No adverse events occurred.
Conclusion: Aflibercept as a first-line therapy was effective and well tolerated for treating clinically significant DME in naïve patients in daily practice. Successful results in terms of improvement of visual and reduction in central macular thickness contribute to provide evidence for the positioning of aflibercept as a first-line indication of newly diagnosed clinically significant DME.
Keywords: aflibercept; central macular thickness; diabetic macular edema; routine clinical practice; visual acuity.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Vision-Related Quality of Life in Patients with Diabetic Macular Edema Treated with Intravitreal Aflibercept: The AQUA Study.Ophthalmol Retina. 2019 Jul;3(7):567-575. doi: 10.1016/j.oret.2019.03.012. Epub 2019 Mar 21. Ophthalmol Retina. 2019. PMID: 31080168 Clinical Trial.
-
Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema.Graefes Arch Clin Exp Ophthalmol. 2017 Jun;255(6):1133-1140. doi: 10.1007/s00417-017-3624-y. Epub 2017 Feb 25. Graefes Arch Clin Exp Ophthalmol. 2017. PMID: 28238195 Clinical Trial.
-
[New perspectives in the approach to diabetic macular edema. Aflibercept therapy].Arch Soc Esp Oftalmol. 2015 Mar;90 Suppl 1:24-8. doi: 10.1016/S0365-6691(15)30006-X. Arch Soc Esp Oftalmol. 2015. PMID: 25925048 Review. Spanish.
-
Clinical Outcomes of a Treat and Extend Regimen with Intravitreal Aflibercept Injections in Patients with Diabetic Macular Edema: Experience in Clinical Practice.Ophthalmol Ther. 2020 Mar;9(1):87-101. doi: 10.1007/s40123-019-00224-x. Epub 2019 Nov 21. Ophthalmol Ther. 2020. PMID: 31755040 Free PMC article.
-
Real-World Evidence in the Management of Diabetic Macular Edema with Intravitreal Anti-VEGFs in Asia: A Systematic Literature Review.Clin Ophthalmol. 2022 Oct 19;16:3503-3526. doi: 10.2147/OPTH.S378392. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36274678 Free PMC article. Review.
Cited by
-
Aflibercept with adjuvant micropulsed yellow laser versus aflibercept monotherapy in diabetic macular edema.Graefes Arch Clin Exp Ophthalmol. 2019 Jul;257(7):1373-1380. doi: 10.1007/s00417-019-04355-6. Epub 2019 May 24. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 31127381 Clinical Trial.
-
Comparison of ranibizumab, aflibercept, and dexamethasone implant monotherapy in treatment-naive eyes with diabetic macular edema: A 12-month real-life experience.Indian J Ophthalmol. 2024 May 1;72(Suppl 3):S453-S458. doi: 10.4103/IJO.IJO_2310_23. Epub 2024 Mar 8. Indian J Ophthalmol. 2024. PMID: 38648453 Free PMC article.
-
Managing Diabetic Macular Edema in Clinical Practice: Systematic Review and Meta-Analysis of Current Strategies and Treatment Options.Clin Ophthalmol. 2021 Jan 29;15:375-385. doi: 10.2147/OPTH.S236423. eCollection 2021. Clin Ophthalmol. 2021. PMID: 33551641 Free PMC article. Review.
-
Efficacy and Safety of Aflibercept Therapy for Diabetic Macular Edema: A Systematic Review and Meta-Analysis.J Curr Ophthalmol. 2022 Jul 26;34(2):133-147. doi: 10.4103/joco.joco_308_21. eCollection 2022 Apr-Jun. J Curr Ophthalmol. 2022. PMID: 36147265 Free PMC article. Review.
-
Switching to ziv-aflibercept in resistant diabetic macular edema non responsive to ranibizumab injection.BMC Ophthalmol. 2022 Jun 29;22(1):287. doi: 10.1186/s12886-022-02503-x. BMC Ophthalmol. 2022. PMID: 35768859 Free PMC article.
References
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. - PubMed
-
- Ding J, Wont TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12(4):346–354. - PubMed
-
- Executive Summary IFD Diabetes Atlas. 7th edition. [Accessed July 9, 2017]. Available from: http://www.diabetesatlas.org/
LinkOut - more resources
Full Text Sources
Other Literature Sources