Renal-protective effect of thalidomide in streptozotocin-induced diabetic rats through anti-inflammatory pathway
- PMID: 29386886
- PMCID: PMC5765978
- DOI: 10.2147/DDDT.S149298
Renal-protective effect of thalidomide in streptozotocin-induced diabetic rats through anti-inflammatory pathway
Abstract
Background: Diabetic nephropathy (DN) is a major microvascular complication in diabetes. An increasing body of evidence has shown that DN is related to chronic inflammation, kidney hypertrophy, and fibrosis. While thalidomide has been shown to have anti-inflammatory and antifibrotic effects, the effects of thalidomide on the pathogenesis of DN are unclear. This study was undertaken to explore whether thalidomide has renal-protective effects in diabetic rats.
Methods: Male Sprague Dawley rats were injected intraperitoneally with 50 mg/kg streptozotocin to induce diabetes. Diabetic rats were treated with thalidomide (200 mg/kg/d) for 8 weeks, and then blood and urine were collected for measurement of renal function-related parameters. Histopathology, immunohistochemistry, enzyme-linked immunosorbent assay, and Western blot analyses were performed to assess renal proinflammatory cytokines, fibrotic protein, and related signaling pathways.
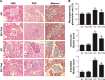
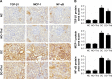
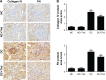
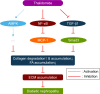
Results: Diabetic rats exhibited obvious renal structural and functional abnormalities, as well as renal inflammation and fibrosis. Compared with diabetic control rats, those treated with thalidomide showed significantly improved histological alterations and biomarkers of renal function, as well as reduced expression of renal inflammatory cytokines, including NF-κB and MCP-1. Furthermore, renal fibrotic proteins, such as TGF-β1, TβRII, TβRI, smad3, collagen IV, and fibronectin were also remarkably suppressed. Treatment with thalidomide markedly stimulated the phosphorylation of AMPKα.
Conclusion: In this study, thalidomide suppressed the inflammatory and fibrotic processes in DN. These effects were partly mediated by the activation of AMPKα, and inhibition of the NF-κB/MCP-1 and TGF-β1/Smad signaling pathways. These results suggest that thalidomide may have therapeutic potential in diabetic renal injury through the anti-inflammatory pathway.
Keywords: AMPK; NF-κB; TGF-β1; diabetic nephropathy; thalidomide.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways.Am J Physiol Renal Physiol. 2017 Aug 1;313(2):F414-F422. doi: 10.1152/ajprenal.00393.2016. Epub 2017 May 31. Am J Physiol Renal Physiol. 2017. PMID: 28566504
-
Terpene glycoside component from Moutan Cortex ameliorates diabetic nephropathy by regulating endoplasmic reticulum stress-related inflammatory responses.J Ethnopharmacol. 2016 Dec 4;193:433-444. doi: 10.1016/j.jep.2016.09.043. Epub 2016 Sep 21. J Ethnopharmacol. 2016. PMID: 27664441
-
Diverse roles of TGF-β receptor II in renal fibrosis and inflammation in vivo and in vitro.J Pathol. 2012 Jun;227(2):175-88. doi: 10.1002/path.3976. Epub 2012 Feb 22. J Pathol. 2012. PMID: 22190171
-
NF-κB pathway as a molecular target for curcumin in diabetes mellitus treatment: Focusing on oxidative stress and inflammation.Cell Biochem Funct. 2024 Jun;42(4):e4030. doi: 10.1002/cbf.4030. Cell Biochem Funct. 2024. PMID: 38720663 Review.
-
Combination therapy with Exendin-4 and islet transplantation as a synergistic treatment for diabetic nephropathy in rats.Life Sci. 2021 Apr 15;271:119207. doi: 10.1016/j.lfs.2021.119207. Epub 2021 Feb 8. Life Sci. 2021. PMID: 33571517 Review.
Cited by
-
Protective Effects of Thalidomide on High-Glucose-Induced Podocyte Injury through In Vitro Modulation of Macrophage M1/M2 Differentiation.J Immunol Res. 2020 Aug 27;2020:8263598. doi: 10.1155/2020/8263598. eCollection 2020. J Immunol Res. 2020. PMID: 32908940 Free PMC article.
-
Targeting Oxidative Stress as a Therapeutic Approach for Idiopathic Pulmonary Fibrosis.Front Pharmacol. 2022 Jan 21;12:794997. doi: 10.3389/fphar.2021.794997. eCollection 2021. Front Pharmacol. 2022. PMID: 35126133 Free PMC article. Review.
-
Therapeutic strategies targeting mechanisms of macrophages in diabetic heart disease.Cardiovasc Diabetol. 2024 May 15;23(1):169. doi: 10.1186/s12933-024-02273-4. Cardiovasc Diabetol. 2024. PMID: 38750502 Free PMC article. Review.
-
Gambogic Acid Mitigates Nephropathy by Inhibiting Oxidative Stress and Inflammation in Diabetic Rats.Int J Mol Cell Med. 2025;14(1):448-461. doi: 10.22088/IJMCM.BUMS.14.1.448. Int J Mol Cell Med. 2025. PMID: 40123583 Free PMC article.
-
Thalidomide interaction with inflammation in idiopathic pulmonary fibrosis.Inflammopharmacology. 2023 Jun;31(3):1167-1182. doi: 10.1007/s10787-023-01193-1. Epub 2023 Mar 25. Inflammopharmacology. 2023. PMID: 36966238 Free PMC article. Review.
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. Available from: https://idf.org/
-
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

