KDM2B overexpression correlates with poor prognosis and regulates glioma cell growth
- PMID: 29386904
- PMCID: PMC5764301
- DOI: 10.2147/OTT.S149833
KDM2B overexpression correlates with poor prognosis and regulates glioma cell growth
Abstract
Background: Gliomas are one of the most lethal cancers in the human central nervous system. Despite clinical treatment advancements, the prognosis of patients with glioma remains poor. KDM2B is a histone lysine demethylase, which has been observed in multiple tumors. But the concrete role of KDM2B in gliomas remains to be further illustrated.
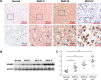
Methods: The KDM2B expression in gliomas was detected with immunohistochemistry and Western blot assay. Furthermore, knockdown of KDM2B in U87 and U251 glioma cell lines, the proliferation capacity was evaluated by cell viability assay, colon formation assay and flow cytometry in vitro. Western blot assay was used to analyze the p21, EZH2 and cyclinD1 changes followed by knockdown of KDM2B.
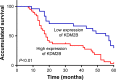
Results: KDM2B was upregulated in tissues of glioma patients, and the expression was correlated to cancer progression. Downregulation of KDM2B in U87 and U251 glioma cell lines inhibited cell proliferation and arrested cell cycle in G0/G1 phase. In addition, silencing KDM2B promoted the upregulation of p21 while reduced the expression of EZH2 and cyclinD1.
Conclusion: Taken together, our results revealed that KDM2B might influence gliomas growth and act as a novel therapeutic target for glioma patients.
Keywords: EZH2; KDM2B; P21; glioma.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
The Role of KDM2B and EZH2 in Regulating the Stemness in Colorectal Cancer Through the PI3K/AKT Pathway.Front Oncol. 2021 Mar 9;11:637298. doi: 10.3389/fonc.2021.637298. eCollection 2021. Front Oncol. 2021. PMID: 33791221 Free PMC article.
-
Histone demethylase KDM2B upregulates histone methyltransferase EZH2 expression and contributes to the progression of ovarian cancer in vitro and in vivo.Onco Targets Ther. 2017 Jun 26;10:3131-3144. doi: 10.2147/OTT.S134784. eCollection 2017. Onco Targets Ther. 2017. PMID: 28706445 Free PMC article.
-
Knockdown of HOXC6 inhibits glioma cell proliferation and induces cell cycle arrest by targeting WIF-1 in vitro and vivo.Pathol Res Pract. 2018 Nov;214(11):1818-1824. doi: 10.1016/j.prp.2018.09.001. Epub 2018 Sep 12. Pathol Res Pract. 2018. PMID: 30228024
-
CBX3 promotes glioma U87 cell proliferation and predicts an unfavorable prognosis.J Neurooncol. 2019 Oct;145(1):35-48. doi: 10.1007/s11060-019-03286-w. Epub 2019 Sep 9. J Neurooncol. 2019. PMID: 31502042
-
CDCA7L promotes glioma proliferation by targeting CCND1 and predicts an unfavorable prognosis.Mol Med Rep. 2019 Aug;20(2):1149-1156. doi: 10.3892/mmr.2019.10349. Epub 2019 Jun 5. Mol Med Rep. 2019. PMID: 31173217 Free PMC article.
Cited by
-
The Role of KDM2B and EZH2 in Regulating the Stemness in Colorectal Cancer Through the PI3K/AKT Pathway.Front Oncol. 2021 Mar 9;11:637298. doi: 10.3389/fonc.2021.637298. eCollection 2021. Front Oncol. 2021. PMID: 33791221 Free PMC article.
-
Latest updates on cellular and molecular biomarkers of gliomas.Front Oncol. 2022 Nov 8;12:1030366. doi: 10.3389/fonc.2022.1030366. eCollection 2022. Front Oncol. 2022. PMID: 36425564 Free PMC article. Review.
-
Lysine Demethylases: Promising Drug Targets in Melanoma and Other Cancers.Front Genet. 2021 Jun 16;12:680633. doi: 10.3389/fgene.2021.680633. eCollection 2021. Front Genet. 2021. PMID: 34220955 Free PMC article. Review.
-
Epigenetic Modifiers: Exploring the Roles of Histone Methyltransferases and Demethylases in Cancer and Neurodegeneration.Biology (Basel). 2024 Dec 3;13(12):1008. doi: 10.3390/biology13121008. Biology (Basel). 2024. PMID: 39765675 Free PMC article. Review.
-
Co-overexpression of RIOK1 and AKT1 as a prognostic risk factor in glioma.J Cancer. 2021 Jul 25;12(19):5745-5752. doi: 10.7150/jca.60596. eCollection 2021. J Cancer. 2021. PMID: 34475988 Free PMC article.
References
-
- Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21(8):1624–1636. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

