Intrauterine device quo vadis? Why intrauterine device use should be revisited particularly in nulliparous women?
- PMID: 29386919
- PMCID: PMC5683133
- DOI: 10.2147/OAJC.S72687
Intrauterine device quo vadis? Why intrauterine device use should be revisited particularly in nulliparous women?
Abstract
Background: Long-acting reversible contraceptive (LARC) methods, including intrauterine devices (IUDs) and the contraceptive implant, are considered the best methods for preventing unintended pregnancies, rapid repeat pregnancy, and abortion in young women. An opinion paper of 2012 by the American College of Obstetricians and Gynecologists recommends Mirena and Paragard for use in nulliparous and adolescent women. However, these IUDs are not designed for young women and are not optimal as they often lead to early discontinuation.
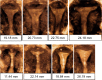
Objective: This article was written with the objective to respond to the urgent need to improve intrauterine contraception as it is likely that the objectives of LARC will not be met without significant improvement of IUD design. Anatomical variations in size and shape of the uterus are not sufficiently considered, producing harm and suffering, which often lead to early removal of the IUD.
Proposed problem solving: The article describes why IUDs should be revisited to meet the challenge of LARC and proposes how to solve these problems. The opinion statement presented here may be considered provocative but is based on hundreds of women with IUD problems who consult or are referred to the practices of the authors of this article due to the disproportion between the IUD and their small uterine cavity. The solution is simple but requires a revision of the current design of IUDs. One-dimensional (longitudinal) IUDs are likely to be the first option. Framed devices with shortened transverse arm and IUDs which adapt to the width of the given uterus are viewed as second best.
Conclusion: One of the reasons of the high unintended pregnancy rate in the USA may be the paucity of suitable IUDs. Also, the legal climate in the USA seems to be a problem for developers as many lawsuits have recently been reported. Clinical studies conducted in young nulliparous and adolescent women suggest that IUDs that fit well in the uterine cavity, like a shoe, result in better tolerance, less side effects, and last but not least, higher use continuation rates.
Keywords: IUD screening; adapted IUDs; continuation rate; counseling; efficacy; frameless IUD/IUS; tolerance.
Conflict of interest statement
Disclosure Dirk Wildemeersch has been involved in the optimization of new, innovative drug delivery systems for use in the uterus. He is currently an advisor in devising new concepts in controlled release for contraception, gynecological treatment, and prevention of infectious diseases. All other authors report no conflicts of interest in this work.
Figures


References
-
- Blumenthal P, Voedisch A, Gemzell-Danielson K. Strategies to prevent unintended pregnancy: increasing use of long-acting reversible contraception. Hum Reprod Update. 2011;17:121–137. - PubMed
-
- Haimovich S. Profile of long-acting reversible contraception users in Europe. Eur J Contracept Reprod Health Care. 2009;14:187–195. - PubMed
-
- American College of Obstetricians and Gynecologists (ACOG) Committee opinion no 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2012;120:983–988. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

